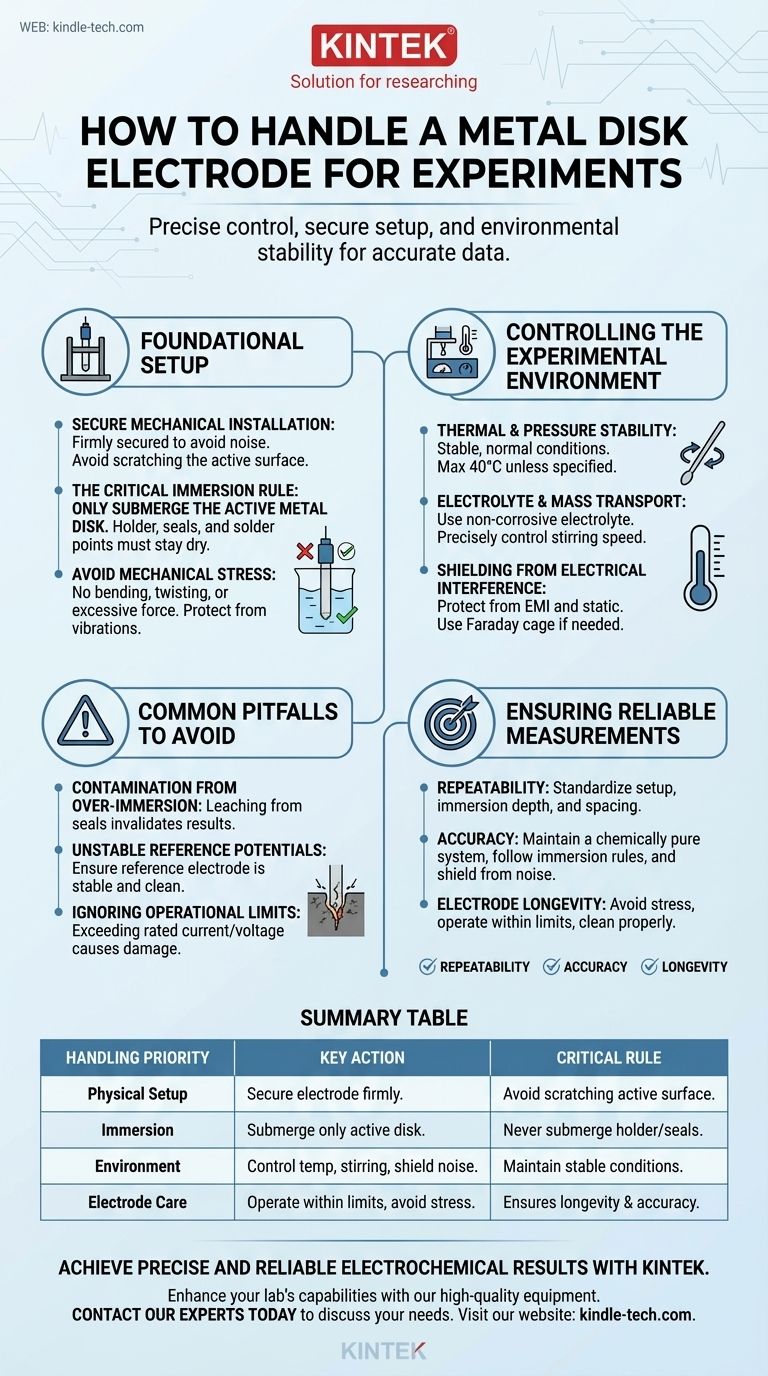
To properly handle a metal disk electrode during an experiment, you must ensure it is installed securely in the electrochemical cell, with only the active disk surface submerged in the electrolyte. Precise control over experimental conditions like temperature and stirring, combined with careful monitoring of electrical signals, is essential for obtaining accurate and repeatable data.
The central principle of handling an electrode is to create a perfectly controlled and isolated system. Your goal is to ensure that the only interaction being measured is between the pristine electrode surface and the bulk electrolyte, free from physical stress, electrical noise, or contamination.

Foundational Setup: Physical and Electrical Integrity
A successful experiment begins with a flawless physical and electrical setup. Any instability introduced at this stage will compromise every subsequent measurement.
Secure Mechanical Installation
The electrode must be firmly secured in its holder or clip. A loose connection can introduce significant electrical noise and inconsistent results.
When tightening the sample, take care not to scratch or deform the active electrode surface, as this alters its electrochemical properties.
Finally, position the working electrode appropriately relative to the reference and counter electrodes, maintaining a consistent distance between them for repeatable measurements.
The Critical Immersion Rule
Only the metal disk surface should be immersed in the electrolyte solution. This is one of the most common and critical errors in practice.
The holder, clip head, and any solder points are sealed with adhesives that are not designed for prolonged chemical exposure.
Submerging these parts can cause the adhesive to fail, leading to contamination of your solution and irreversible damage to the electrode's internal connections.
Avoiding Mechanical Stress
The electrode assembly is a sensitive instrument. Never subject it to bending, twisting, or other significant mechanical forces.
Furthermore, protect the experimental apparatus from external mechanical vibrations, as these can disturb the diffusion layer at the electrode surface and affect current measurements.
Controlling the Experimental Environment
Your electrochemical cell does not exist in a vacuum. The surrounding environment must be carefully managed to ensure the conditions of your measurement are stable and known.
Thermal and Pressure Stability
For most applications, experiments should be conducted at a stable, normal temperature and pressure. A maximum operating temperature of 40°C is a safe guideline unless specified otherwise.
If your experiment requires temperature control, you may use a water bath, but you must still adhere to the critical immersion rule: only the active electrode surface may contact the heating or cooling medium.
Electrolyte and Mass Transport
Ensure your chosen electrolyte is non-corrosive and non-reactive with both the electrode disk and any part of the holder that might be exposed to fumes.
Stirring speed must be precisely controlled. This parameter directly governs mass transport to the electrode surface and is a critical variable in many electrochemical techniques.
Shielding from Electrical Interference
Electrochemical signals, especially low currents, are highly susceptible to external noise.
Protect your setup from electromagnetic interference (EMI) and static electricity. If you are working in an electrically noisy environment, using a Faraday cage or other shielding and grounding techniques is essential for signal accuracy.
Common Pitfalls to Avoid
Trustworthy data comes from understanding what can go wrong. Being aware of these common failure points is critical for troubleshooting and validation.
Contamination from Over-Immersion
The most frequent mistake is submerging the electrode holder along with the active surface. This introduces unknown variables into your experiment as materials leach from the holder's seals and solder points, invalidating your results.
Unstable Reference Potentials
While your focus is on the working electrode, its potential is measured against a reference electrode. If the reference electrode is unstable, clogged, or improperly prepared, all of your potential measurements will be meaningless. Always ensure your reference is stable before starting.
Ignoring Operational Limits
Every electrode has rated current and voltage limits. Exceeding these can cause polarization effects, where the electrode surface is irreversibly altered, or can lead to damage. Always operate within the manufacturer's specified range.
How to Ensure Reliable Measurements
Your experimental protocol should be designed to produce results that are accurate, repeatable, and defendable.
- If your primary focus is repeatability: Standardize your physical setup by ensuring the immersion depth and electrode spacing are identical for every run.
- If your primary focus is accuracy: Prioritize a chemically pure system by strictly following the immersion rule and shielding your cell from all sources of electrical noise.
- If your primary focus is electrode longevity: Avoid all mechanical stress, operate well within the recommended temperature and electrical limits, and clean the electrode properly after each use.
Meticulous and consistent handling is the absolute foundation of trustworthy electrochemical data.
Summary Table:
| Handling Priority | Key Action | Critical Rule |
|---|---|---|
| Physical Setup | Secure electrode firmly in holder. | Avoid scratching the active disk surface. |
| Immersion | Submerge only the active metal disk. | Never submerge the holder or seals to prevent contamination. |
| Environment | Control temperature, stirring, and shield from electrical noise. | Maintain stable conditions for repeatable measurements. |
| Electrode Care | Operate within current/voltage limits and avoid mechanical stress. | Ensures electrode longevity and data accuracy. |
Achieve precise and reliable electrochemical results with the right equipment.
Proper electrode handling is just one part of a successful experiment. KINTEK specializes in high-quality lab equipment and consumables, providing the reliable tools your laboratory needs for accurate electrochemical analysis.
Let us help you enhance your lab's capabilities. Contact our experts today to discuss your specific requirements and discover how our solutions can support your research goals.
Visual Guide
Related Products
- Metal Disc Electrode Electrochemical Electrode
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
- Gold Electrochemical Sheet Electrode Gold Electrode
- Gold Disc Electrode
People Also Ask
- What is the common role of a platinum disk electrode? A Guide to Its Primary Use as a Working Electrode
- What is the proper post-experiment procedure for a metal disk electrode? Ensure Accurate, Reproducible Results
- What materials can be used for metal disk electrodes? Selecting the Right Metal for Your Electrochemical Experiment
- What is the expected lifespan of a metal disk electrode? Extend Its Life with Proper Care
- How should a metal disk electrode be maintained? A Guide to Consistent, Reliable Electrochemical Data



















