Operating an all-PTFE electrolytic cell requires a systematic approach focused on three core areas: meticulous preparation, precise control during the experiment, and unwavering adherence to safety protocols. This involves carefully inspecting the cell and electrodes, preparing high-purity electrolytes, strictly managing parameters like voltage and temperature, and actively monitoring the reaction for any visual or data anomalies.
The key to a successful experiment is understanding that an all-PTFE cell’s chemical inertness is an advantage that must be protected. Success hinges not just on following steps, but on maintaining a pristine and controlled environment from start to finish to ensure both data integrity and personal safety.
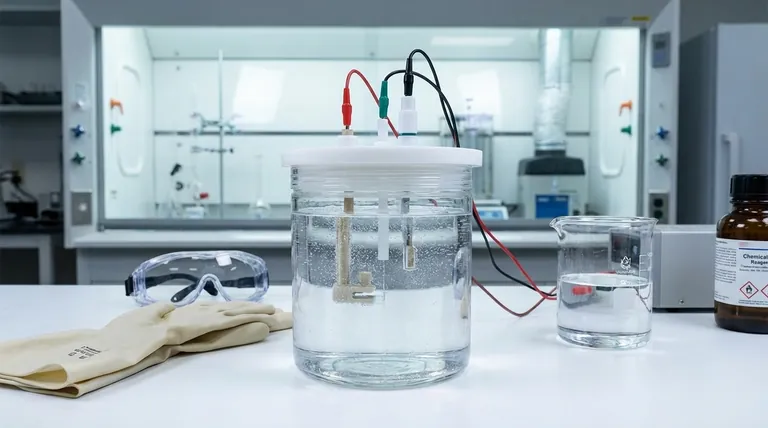
Pre-Experiment Preparation and Inspection
Before any power is applied, the foundation for a reliable experiment is laid. Rushing this stage is a common source of error.
Inspecting Cell Integrity
You must visually inspect the PTFE cell body for any signs of cracks, stress whitening, or leaks, especially around seals and fittings. Although PTFE is robust, physical damage can compromise the experiment and create safety hazards.
Verifying Electrode Condition
Ensure the electrode surfaces are clean, polished, and free from any prior residue or physical damage. The state of the electrode surface is critical as it is the direct interface for the electrochemical reaction.
Preparing the Electrolyte
Use only high-purity chemical reagents and deionized or distilled water. Impurities in the electrolyte are a primary source of experimental artifacts and can lead to unintended side reactions.
Executing the Experiment with Precision
During operation, your role shifts from preparation to active monitoring and control. The cell will provide constant feedback if you know what to look for.
Filling the Cell
Slowly pour the prepared electrolyte into the cell, taking care to avoid splashing or introducing excessive air bubbles. Never fill the cell beyond 80% of its maximum volume to provide a buffer against splashing or thermal expansion.
Controlling Key Parameters
All experimental parameters—voltage, current, temperature, and flow rate—must be strictly controlled and monitored. These variables directly influence the reaction rate and outcome, and deviation can lead to inconsistent results or damage the cell.
Actively Monitoring the Reaction
Closely observe the process. Pay attention to the rate and nature of bubble formation on the electrodes, any color changes in the electrolyte, and fluctuations in temperature. These are real-time indicators of the reaction's health.
Recording Data Systematically
Keep a detailed log of the electrolysis time, temperature, and any changes observed in the electrolyte's state. If instrument data or curves show any anomalies, be prepared to pause the experiment to diagnose the issue.
Critical Safety Protocols
The chemical inertness of PTFE does not eliminate the inherent risks of electrochemical work. The hazards come from the electrolytes, the electrical energy, and the potential byproducts.
Personal Protective Equipment (PPE)
Always wear appropriate PPE, including acid and alkali-resistant gloves and safety goggles. Direct contact with many electrolytes can cause severe chemical burns.
Managing Hazardous Byproducts
Ensure the experiment is conducted in a well-ventilated area, such as a fume hood. Electrolysis can produce harmful or flammable gases (e.g., chlorine, hydrogen), and proper ventilation is essential to prevent their accumulation.
Electrical and Chemical Safety
Never touch the electrodes or electrolyte while the power supply is active to prevent electric shock. Keep all open flames or flammable materials far away from the experimental setup.
Common Pitfalls to Avoid
Even with a perfect procedure, a lack of awareness of common failure points can undermine your results.
Overlooking Contamination
The most common pitfall is subtle contamination. Using insufficiently pure reagents or failing to properly clean the cell and electrodes between runs will introduce variables that corrupt your data.
Ignoring Visual Cues
Changes in bubble formation or electrolyte color are not just minor observations; they are critical data points. Ignoring them can mean missing the onset of an unwanted side reaction or electrode degradation.
Prolonged Overload Operation
Running the cell at maximum current or voltage for extended periods can cause excessive heat, degrading the electrodes, the electrolyte, and potentially the PTFE cell itself. Adhere to the recommended operating limits of your equipment.
Making the Right Choice for Your Goal
Your operational focus should align with your primary experimental objective.
- If your primary focus is data accuracy: Prioritize the purity of your electrolyte and the cleanliness of your electrodes above all else.
- If your primary focus is operational safety: Double-check your ventilation setup and ensure all personal protective equipment is worn correctly before starting.
- If your primary focus is equipment longevity: Avoid prolonged overload operation and perform a thorough inspection of the cell before and after every use.
Ultimately, a disciplined and observant approach transforms a routine procedure into a reliable and repeatable scientific experiment.
Summary Table:
| Stage | Key Actions | Critical Considerations |
|---|---|---|
| Preparation | Inspect cell integrity, verify electrode condition, prepare high-purity electrolyte | Avoid contamination; ensure no physical damage to PTFE or electrodes |
| Execution | Control voltage/current/temperature, monitor bubble formation/color changes, record data systematically | Never fill beyond 80% volume; watch for anomalies in real-time |
| Safety | Wear acid/alkali-resistant gloves and goggles, use a fume hood, avoid open flames | Electrolytes and byproducts (e.g., gases) pose chemical and electrical hazards |
Optimize Your Electrochemical Experiments with KINTEK
Achieve consistent, high-integrity results in your lab with reliable equipment and expert support. KINTEK specializes in high-quality lab equipment and consumables, including electrolytic cells designed for precision and durability.
Let us help you enhance your experimental outcomes—contact our experts today to discuss your specific laboratory needs and discover the right solutions for your research.
Visual Guide

Related Products
- Electrolytic Electrochemical Cell with Five-Port
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell Gas Diffusion Liquid Flow Reaction Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
People Also Ask
- What are the standard components of the five-port water bath electrolytic cell? Master the Precision Instrument for Electrochemical Analysis
- How should the body of an electrolytic cell be maintained for longevity? Extend Your Equipment's Lifespan
- What are the proper storage procedures for the multifunctional electrolytic cell? Protect Your Investment and Ensure Data Accuracy
- What general precaution should be taken when handling the electrolytic cell? Ensure Safe and Accurate Lab Results
- How can leaks be prevented when using a five-port water bath electrolytic cell? Ensure a Reliable and Safe Electrochemical Setup



















