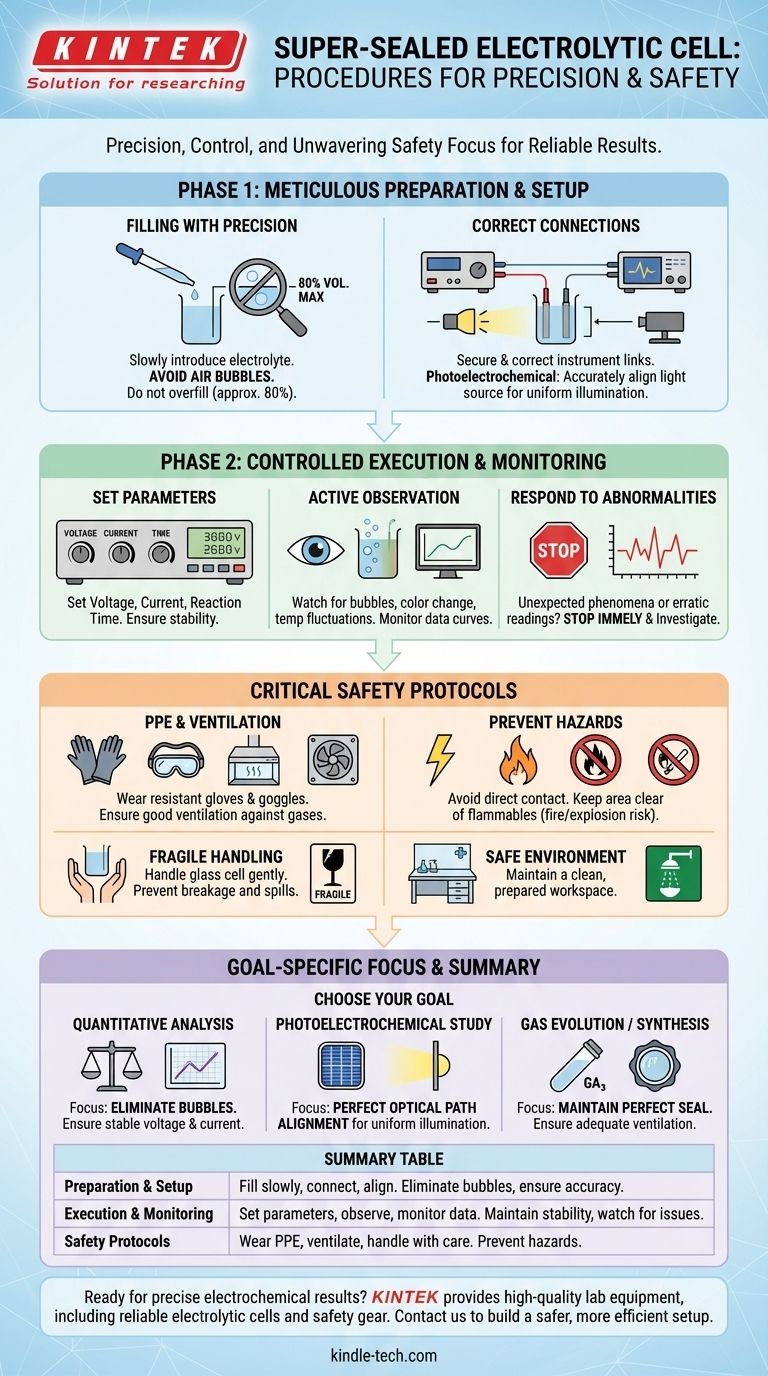
Following the correct procedure with a super-sealed electrolytic cell is a matter of precision, control, and unwavering attention to safety. The core process involves carefully filling the cell to avoid interference, connecting instrumentation accurately, controlling reaction parameters like voltage and current, and actively monitoring the experiment for any changes or abnormalities.
The goal is not simply to follow a checklist, but to create a highly controlled environment. Every step, from eliminating air bubbles to ensuring proper ventilation, is designed to protect the integrity of your data, the longevity of your equipment, and your personal safety.

Phase 1: Meticulous Preparation and Setup
Before the reaction begins, the setup phase is critical for ensuring the experiment is valid and repeatable. Errors made here will compromise all subsequent data.
Filling the Cell with Precision
Slowly introduce the prepared electrolyte into the cell's opening. The primary goal is to avoid introducing or forming air bubbles, which can block the electrode surface and interfere with results.
If bubbles do appear, gently tap the cell to dislodge them. Be careful not to fill the cell beyond its maximum capacity, typically around 80% of its total volume, to prevent splashing during handling or gas evolution.
Ensuring Correct Instrumentation Connections
Connect the electrolytic cell to the power supply and any detection instruments according to the experimental design. Double-check that all connections are secure and correct.
For photoelectrochemical experiments, this step also involves accurately aligning the light source with the cell's side window. The optical path must be adjusted to ensure the light uniformly illuminates the entire working electrode surface.
Phase 2: Controlled Execution and Monitoring
With the cell prepared, the focus shifts to running the experiment while maintaining control and observing the process closely.
Setting and Maintaining Key Parameters
Set the required experimental parameters on your instruments. These most often include voltage, current, and reaction time.
Once the experiment starts, verify that the current and voltage are stable and within the expected range. Avoid prolonged operation at overload capacity to prevent damage to the cell or electrodes.
The Importance of Active Observation
Vigilant monitoring is crucial for both data collection and safety. Pay close attention to the phenomena inside the cell.
Look for key indicators such as gas bubble formation on the electrode surfaces, any changes in the electrolyte's color, or noticeable temperature fluctuations. Concurrently, monitor the data and curves being generated by your instruments.
Responding to Abnormalities
If you observe any unexpected phenomena or if the instrument readings become erratic, stop the experiment immediately. Investigating the abnormality is more important than continuing to collect potentially invalid data.
Understanding the Critical Safety Protocols
A super-sealed cell is often used because the reaction may involve hazardous materials or produce dangerous byproducts. Safety cannot be an afterthought.
Personal and Environmental Protection
Always wear appropriate Personal Protective Equipment (PPE), including acid- and alkali-resistant gloves and safety goggles, to protect against chemical splashes.
Ensure the experiment is conducted in an area with good ventilation. Electrolysis can produce harmful or flammable gases (e.g., chlorine, hydrogen), and a sealed cell is designed to contain them, but proper ventilation is a critical secondary precaution.
Preventing Electrical and Chemical Hazards
Never make direct contact with the electrodes or the electrolyte during operation to prevent electric shock or chemical burns.
Keep the area around the electrolytic cell clear of open flames or other flammable materials. The production of gases like hydrogen creates a significant risk of fire or explosion.
Handling the Equipment
Remember that the cell body is typically made of glass and is fragile. Handle it gently and carefully at all times to prevent breakage, which could lead to chemical spills and equipment loss.
Making the Right Choice for Your Goal
Your specific experimental goal will determine which procedural steps require the most focus.
- If your primary focus is quantitative analysis: Your highest priority is eliminating all bubbles and ensuring absolutely stable voltage and current for data accuracy.
- If your primary focus is a photoelectrochemical study: Your critical step is the perfect alignment of the optical path to ensure uniform illumination of the electrode.
- If your primary focus is gas evolution or synthesis: Your most important consideration is maintaining a perfect seal and ensuring adequate ventilation as a safety backup.
By adopting this systematic and safety-conscious approach, you ensure your experimental procedure is a robust framework for generating reliable and meaningful results.
Summary Table:
| Phase | Key Steps | Critical Focus |
|---|---|---|
| Preparation & Setup | Fill cell slowly, connect instruments, align light source. | Eliminate air bubbles, ensure accurate connections. |
| Execution & Monitoring | Set voltage/current, observe reactions, monitor data. | Maintain stable parameters, watch for abnormalities. |
| Safety Protocols | Wear PPE, ensure ventilation, handle with care. | Prevent chemical, electrical, and explosion hazards. |
Ready to achieve precise and safe electrochemical results? The right equipment is fundamental to your success. KINTEK specializes in high-quality lab equipment and consumables, including reliable electrolytic cells and safety gear, designed to meet the rigorous demands of your laboratory. Let our experts help you build a safer, more efficient setup. Contact KINTEK today to discuss your specific needs and enhance your experimental outcomes!
Visual Guide
Related Products
- Electrolytic Electrochemical Cell with Five-Port
- H Type Electrolytic Cell Triple Electrochemical Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
- Thin-Layer Spectral Electrolysis Electrochemical Cell
People Also Ask
- How can contamination be avoided during experiments with the five-port water bath electrolytic cell? Master the 3-Pillar Protocol
- What are the standard components of the five-port water bath electrolytic cell? Master the Precision Instrument for Electrochemical Analysis
- What are the proper storage procedures for the multifunctional electrolytic cell? Protect Your Investment and Ensure Data Accuracy
- What general precaution should be taken when handling the electrolytic cell? Ensure Safe and Accurate Lab Results
- How should the five-port water bath electrolytic cell be cleaned for maintenance? A Step-by-Step Guide to Reliable Results



















