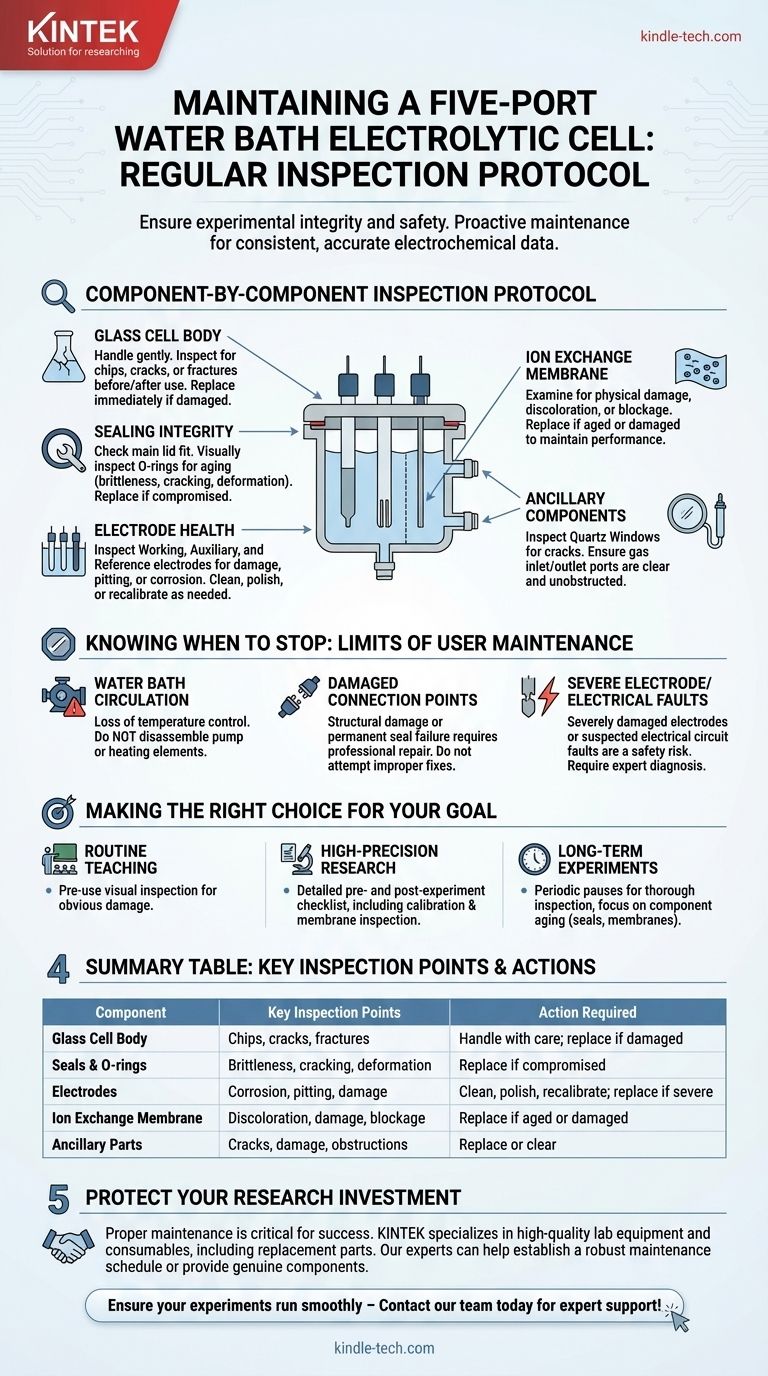
To properly maintain a five-port water bath electrolytic cell, you must perform regular visual inspections of its core components. This includes checking the glass cell body for cracks, ensuring all seals and O-rings are intact, examining the electrodes for signs of corrosion or damage, and inspecting the ion exchange membrane for any aging or blockage. Any component found to be damaged must be replaced immediately to ensure experimental integrity and safety.
The goal of regular inspection is not just to prevent failure, but to guarantee the consistency and accuracy of your electrochemical data. Proactive maintenance is the foundation of reproducible results.

A Component-by-Component Inspection Protocol
Effective maintenance relies on a systematic check of each part of the cell assembly. Each component has a specific function and a unique set of potential failure points.
The Glass Cell Body
The cell body itself is the primary containment vessel. As it is made of glass, it is inherently fragile.
Always handle the cell gently to prevent breakage. Before and after each use, inspect the body for any chips, cracks, or fractures, as these can compromise the experiment and become a safety hazard.
Sealing Integrity
A perfect seal is critical for preventing leaks, maintaining an inert atmosphere, and avoiding contamination.
Check the main cell lid to ensure it fits tightly and securely. Visually inspect all sealing rings (O-rings) for signs of aging, such as brittleness, cracking, or deformation, and replace them if they are compromised.
Electrode Health
The electrodes are the heart of your electrochemical experiment, and their condition directly impacts your results.
Inspect the working, auxiliary, and reference electrodes for any physical damage, pitting, or corrosion. Depending on usage, electrodes may require periodic cleaning, polishing, or recalibration to ensure accurate performance.
The Ion Exchange Membrane
The membrane selectively allows ions to pass between cell compartments, and its condition is crucial for many experiments.
Carefully examine the ion exchange membrane for any physical damage, discoloration, or signs of blockage. An aged or damaged membrane can severely alter the cell's performance and must be replaced.
Ancillary Components
Depending on your setup, you may have other critical parts.
If your cell is equipped with quartz windows, inspect them for cracks or damage that could affect optical measurements. Check that any gas inlet or outlet ports are clear and unobstructed.
Knowing When to Stop: The Limits of User Maintenance
While routine inspection and replacement of simple components are expected, certain issues require specialized knowledge. Attempting to fix complex systems yourself can cause further damage.
Water Bath Circulation
If the water bath circulation system is malfunctioning, it can lead to a complete loss of temperature control. Do not attempt to disassemble the pump or heating elements.
Damaged Connection Points
Structural damage to the cell's core connection points or permanent seals requires professional repair. Improper fixes can lead to persistent leaks or electrical faults.
Severe Electrode or Electrical Faults
While minor cleaning is routine, severely damaged electrodes or any suspected electrical circuit faults are beyond the scope of standard maintenance. These issues present a safety risk and require expert diagnosis.
Making the Right Choice for Your Goal
Your maintenance schedule and rigor should match the demands of your work.
- If your primary focus is routine teaching or qualitative analysis: A pre-use visual inspection for obvious damage to the glass, seals, and electrodes is sufficient.
- If your primary focus is high-precision quantitative research: Implement a detailed pre- and post-experiment checklist, including electrode calibration and membrane inspection.
- If your primary focus is long-term or automated experiments: Periodically pause the experiment for a thorough inspection, paying close attention to component aging, especially of seals and membranes.
Consistent, careful inspection is the simplest way to protect your equipment and the validity of your research.
Summary Table:
| Component | Key Inspection Points | Action Required |
|---|---|---|
| Glass Cell Body | Chips, cracks, fractures | Handle with care; replace if damaged |
| Seals & O-rings | Brittleness, cracking, deformation | Replace if compromised to prevent leaks |
| Electrodes | Corrosion, pitting, physical damage | Clean, polish, or recalibrate; replace if severe |
| Ion Exchange Membrane | Discoloration, damage, blockage | Replace if aged or damaged |
| Ancillary Parts (e.g., Quartz Windows) | Cracks, damage, obstructions | Replace or clear to maintain function |
Protect your research investment and ensure data reproducibility. Proper maintenance of your electrolytic cell is critical for success. KINTEK specializes in high-quality lab equipment and consumables, including replacement parts for electrolytic cells. Our experts can help you establish a robust maintenance schedule or provide the genuine components you need.
Ensure your experiments run smoothly – Contact our team today for expert support!
Visual Guide
Related Products
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell with Five-Port
- Multifunctional Electrolytic Electrochemical Cell Water Bath Single Layer Double Layer
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
People Also Ask
- What are the standard opening specifications for an H-type exchangeable membrane electrolytic cell? Asymmetrical Ports for Precise Electrochemistry
- How does the design of an electrolytic cell facilitate the electrochemical regeneration of graphene-based adsorbents?
- What are the key features of the five-port water bath electrolytic cell? Precision Control for Electrochemical Experiments
- What material is the body of the electrolysis cell made of? High Borosilicate Glass for Reliable Electrochemistry
- How do benchtop double-chamber electrolytic cells assist in evaluating the stability of new electrocatalysts?
- How are electrolytic cells and electrochemical workstations used for Pt/Pd fuel cell evaluation? Expert Guide
- Can the material of the electrode clamp in the in-situ Raman electrolytic cell be customized? Tailor Your Research.
- What is the functional design of a laboratory three-electrode electrolytic cell? Precision for Titanium Alloys



















