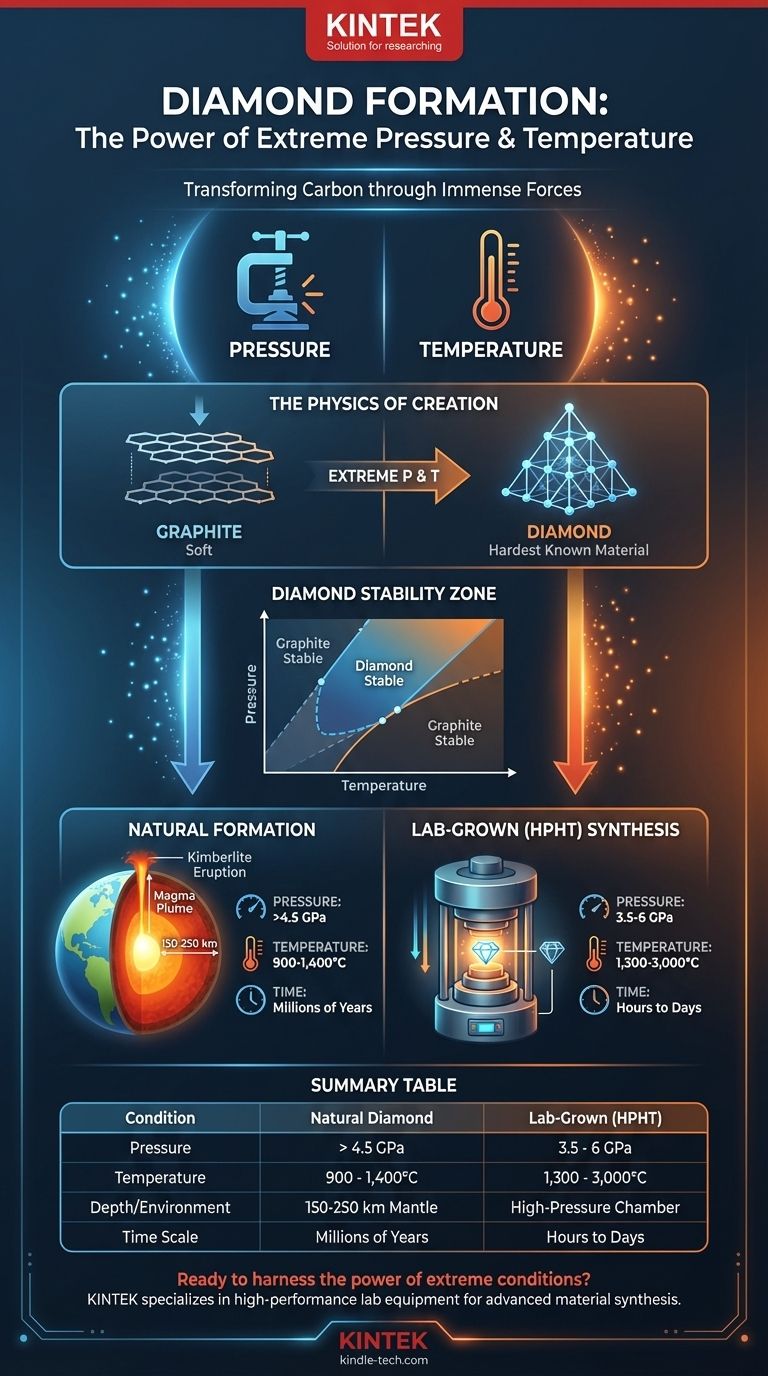
In short, creating a diamond requires immense pressure and extreme temperatures, conditions that are fundamentally different from those on the Earth's surface. For natural diamonds, this means pressures above 4.5 gigapascals (GPa) and temperatures over 900°C, conditions found at depths of 150 kilometers (about 93 miles) or more within the Earth's mantle. Lab-grown methods replicate this, with some processes using pressures of 3.5 GPa and temperatures soaring to 3,000°C to accelerate the transformation.
The immense heat and pressure required for diamond formation are not arbitrary; they are the specific conditions needed to force carbon atoms out of their common graphite structure and into the exceptionally strong and stable tetrahedral lattice that defines a diamond.

The Physics of Diamond Creation
To understand why these conditions are necessary, we must look at carbon's atomic structure. Both graphite (the "lead" in a pencil) and diamond are made of pure carbon, but their properties are vastly different.
Why High Pressure is Non-Negotiable
Graphite is the stable form of carbon at surface-level pressure. Its atoms are arranged in flat sheets that easily slide past one another, making it soft.
Diamond formation requires pressures so intense—over 500,000 times the pressure at sea level—that they physically force carbon atoms closer together. This pressure overcomes the natural arrangement of graphite and compels the atoms to bond in a rigid, three-dimensional tetrahedral lattice. This structure is what gives diamond its unparalleled hardness.
The Role of Extreme Temperature
Pressure alone isn't enough. Extreme temperature, typically above 900°C (1,650°F), provides the atomic energy needed for the transformation to occur.
Heat allows the strong bonds in the source carbon to break, freeing the atoms to move and rearrange themselves. In this high-energy state, they can then lock into the more compact and stable diamond structure dictated by the high-pressure environment.
The Diamond Stability Zone
The combination of pressure and temperature creates a specific environment known as the diamond stability zone. This is a range of conditions, primarily found in the Earth's upper mantle, where diamond is the most thermodynamically stable form of carbon.
Outside of this zone, carbon will either remain as graphite or, if a diamond is brought to the surface too slowly, revert back to graphite.
Where Do These Conditions Occur?
These extreme conditions are found in only two places: deep within the Earth and inside highly specialized laboratory machines.
Natural Formation in the Earth's Mantle
Nearly all natural diamonds formed millions to billions of years ago in the diamond stability zone, roughly 150 to 250 kilometers below the surface.
These crystals were then carried rapidly to the surface during deep-source volcanic eruptions. The magma from these eruptions, known as kimberlite, acted as a high-speed elevator, bringing the diamonds to the surface quickly enough that they did not have time to degrade back into graphite.
Lab-Grown Synthesis (HPHT Method)
Scientists replicate these conditions using the High-Pressure/High-Temperature (HPHT) method. A small diamond seed is placed in a chamber with a source of pure carbon.
The chamber is subjected to immense pressure (often 3.5-6 GPa) and heated to extreme temperatures (1,300-3,000°C). Under these conditions, the source carbon dissolves and re-crystallizes onto the diamond seed, growing a new, larger diamond over hours or days.
Understanding the Trade-offs and Nuances
The process is more complex than a single temperature and pressure recipe. The relationship between the variables is critical.
It's a Window, Not a Single Point
There is no single number for diamond formation. Instead, it is a range of conditions. For instance, formation can occur at a slightly lower pressure if the temperature is significantly higher, and vice versa, as long as the combination falls within the diamond stability zone.
Time is a Critical Factor
Natural diamonds form over geological timescales, allowing them to grow slowly under relatively "cooler" mantle temperatures (around 900-1,400°C).
Lab processes dramatically accelerate this. By using much higher temperatures, sometimes double that of natural formation, scientists can catalyze the transformation and grow a diamond in a fraction of the time. This is a direct trade-off: more heat equals faster growth.
The Myth of Coal
A common misconception is that diamonds are formed from compressed coal. This is incorrect. The vast majority of diamonds are formed from carbon that was trapped in the Earth's mantle since the planet's formation, long before the first land plants—the source of coal—ever existed.
Making the Right Choice for Your Goal
Understanding the conditions of diamond formation helps clarify the difference between natural and synthetic stones and the science that unites them.
- If your primary focus is natural diamonds: The key takeaway is their origin in the deep Earth's mantle (150km+), where they formed over millions of years and were brought to the surface by rare volcanic events.
- If your primary focus is synthetic diamonds: The key takeaway is the use of advanced technology to replicate and often intensify natural conditions to grow a chemically identical diamond in a controlled, accelerated process.
- If your primary focus is the underlying science: The key takeaway is the concept of the "diamond stability zone," a specific pressure-temperature window where carbon atoms are forced into a fundamentally different and more durable atomic structure.
Ultimately, every diamond, whether natural or lab-grown, is a testament to the transformative power of extreme heat and pressure.
Summary Table:
| Condition | Natural Diamond Formation | Lab-Grown (HPHT) Diamond |
|---|---|---|
| Pressure | > 4.5 GPa | 3.5 - 6 GPa |
| Temperature | 900 - 1,400°C | 1,300 - 3,000°C |
| Depth / Environment | 150-250 km in Earth's mantle | Specialized high-pressure chamber |
| Time Scale | Millions of years | Hours to days |
Ready to harness the power of extreme conditions in your own lab? KINTEK specializes in high-performance lab equipment, including systems capable of replicating the intense environments needed for advanced material synthesis. Whether you're researching diamond growth or other high-pressure/high-temperature processes, our expertise and reliable equipment are here to support your groundbreaking work. Contact our experts today to discuss how we can meet your specific laboratory needs.
Visual Guide
Related Products
- Manual High Temperature Heated Hydraulic Press Machine with Heated Plates for Lab
- Automatic High Temperature Heated Hydraulic Press Machine with Heated Plates for Lab
- Warm Isostatic Press WIP Workstation 300Mpa for High Pressure Applications
- 24T 30T 60T Heated Hydraulic Press Machine with Heated Plates for Laboratory Hot Press
- Heated Hydraulic Press Machine with Heated Plates for Vacuum Box Laboratory Hot Press
People Also Ask
- Does a hydraulic press have heat? How Heated Platens Unlock Advanced Molding and Curing
- What does a hydraulic heat press do? Achieve Industrial-Scale, Consistent Pressure for High-Volume Production
- What are heated hydraulic presses used for? Molding Composites, Vulcanizing Rubber, and More
- What is a heated hydraulic press used for? Essential Tool for Curing, Molding, and Laminating
- What causes hydraulic pressure spikes? Prevent System Damage from Hydraulic Shock



















