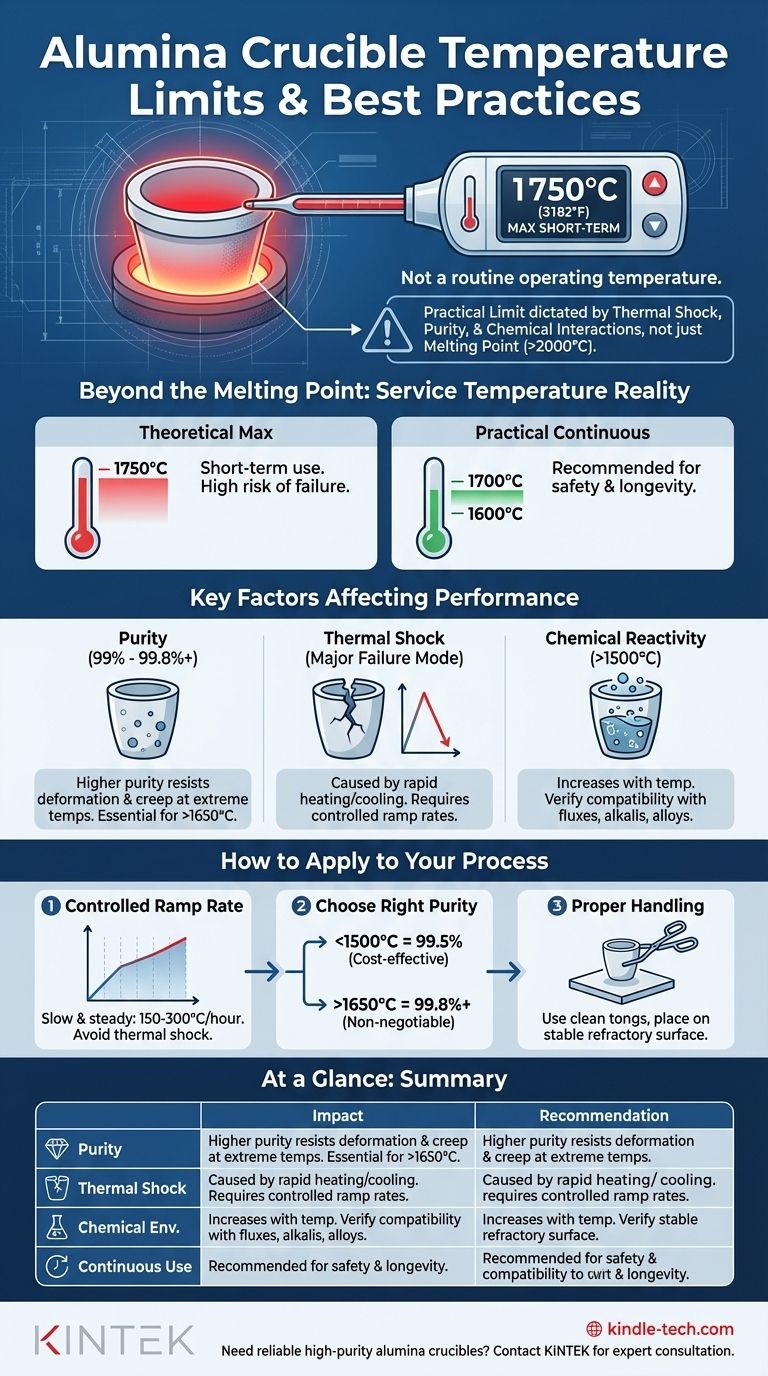
In practice, a high-purity alumina (Al₂O₃) crucible can be used for applications at temperatures up to 1750°C (3182°F). However, this number represents an upper limit under ideal conditions, not a routine operating temperature. The actual service limit is dictated by factors like material purity, the rate of temperature change, and chemical interactions.
The theoretical maximum temperature is a useful guide, but the true key to success with alumina crucibles is understanding that their practical limit is determined by thermal shock resistance and chemical purity, not just their melting point.

Beyond the Melting Point: Understanding Service Temperature
The melting point of pure alumina is over 2000°C, yet its maximum service temperature is rated lower. This gap is crucial to understand for safe and effective use.
The Theoretical Maximum vs. Practical Use
The 1750°C figure is a maximum short-term working temperature for high-purity alumina. Continuously operating at this temperature significantly reduces the crucible's lifespan and increases the risk of failure.
A more conservative and realistic continuous operating temperature is often in the 1600°C to 1700°C range to provide a margin of safety.
The Critical Role of Purity
Alumina crucibles are available in various purities, typically from 99% to 99.8%+. This percentage is the single most important factor in high-temperature performance.
Impurities, such as silica (SiO₂) and other oxides, form glassy phases at high temperatures. These phases soften and lower the temperature at which the crucible itself begins to deform, a process known as creep.
Therefore, the higher the purity, the better the crucible will resist deformation and maintain its structural integrity near its maximum service temperature.
Thermal Shock: The Most Common Failure Mode
Thermal shock is stress induced in a material by a rapid change in temperature. Alumina has good thermal stability, but it is a brittle ceramic and will crack if heated or cooled too quickly.
This is the most common reason for crucible failure. Rapidly inserting a cold crucible into a red-hot furnace or removing a hot crucible into open, cool air is a primary cause of cracking.
Understanding the Trade-offs and Potential Failures
Using any material at its limits involves trade-offs. For alumina, you are primarily balancing speed and cost against reliability and longevity.
Risk of Thermal Shock Cracking
This cannot be overstated. The faster you heat or cool, the higher the risk of fracture. A controlled ramp rate is not a suggestion; it is a requirement for high-temperature work.
Chemical Reactivity
Alumina is exceptionally inert to most chemicals, which is a primary reason for its use. However, at extreme temperatures (above 1500°C), its reactivity increases.
It can be attacked by highly basic fluxes, molten alkali metals, and certain metal alloys. Always verify the chemical compatibility of your sample with Al₂O₃ at your target temperature to avoid contaminating your material or damaging the crucible.
Creep and Deformation
Even below its melting point, a crucible can slowly deform under load at very high temperatures. For applications involving heavy melts held at temperatures above 1600°C for extended periods, using the highest purity alumina is essential to minimize creep.
How to Apply This to Your Process
To prevent failure and ensure a long service life, you must control the operational environment of the crucible.
Follow a Controlled Heating and Cooling Ramp
A slow and steady ramp rate is the best defense against thermal shock. While the ideal rate depends on crucible size and furnace type, a general guideline is 150-300°C per hour. Never place a crucible directly into a pre-heated furnace above a few hundred degrees Celsius.
Choose the Right Purity for the Job
Don't over-spec or under-spec your crucible. If your process runs at 1400°C, a standard 99.5% purity crucible is likely a cost-effective and reliable choice. If you are pushing toward 1700°C, investing in 99.8%+ purity is non-negotiable.
Ensure Proper Handling and Placement
Always handle crucibles with clean tongs to avoid introducing contaminants. Inside the furnace, place the crucible on a flat, stable refractory surface (like an alumina or zirconia plate) to ensure it is evenly supported.
Making the Right Choice for Your Application
Your goal determines how you should approach the temperature limits of alumina.
- If your primary focus is operating near the maximum temperature (>1650°C): You must use the highest purity alumina (99.8%+) and adhere to very strict, slow heating and cooling protocols.
- If your primary focus is general purpose melting or analysis (below 1500°C): A standard purity (99.5%) crucible offers a great balance of performance and cost, with a lower risk of failure.
- If your primary focus is preventing sample contamination: High-purity alumina is essential, and you must verify its chemical inertness with your specific materials at your target operating temperature.
By treating the maximum temperature as a limit to approach with caution rather than a target to hit, you ensure the crucible performs as a reliable tool for your work.
Summary Table:
| Key Factor | Impact on Temperature Limit | Recommendation |
|---|---|---|
| Purity | Higher purity (>99.8%) resists deformation and creep at extreme temperatures. | Use higher purity for >1650°C applications. |
| Thermal Shock | Rapid temperature changes are the most common cause of failure. | Follow a controlled ramp rate of 150-300°C/hour. |
| Chemical Environment | Reactivity increases above 1500°C with certain fluxes and metals. | Verify chemical compatibility with your sample. |
| Continuous Use | Operating at the maximum limit (1750°C) shortens lifespan. | For long-term use, aim for 1600°C to 1700°C. |
Need a reliable alumina crucible for your high-temperature application? KINTEK specializes in high-purity lab equipment, including alumina crucibles tailored for demanding processes up to 1750°C. Our experts can help you select the right purity and specifications to ensure safety, prevent contamination, and extend crucible life. Contact our team today for a personalized consultation and enhance your lab's performance with the right tools.
Visual Guide
Related Products
- Engineering Advanced Fine Ceramics Alumina Crucibles (Al2O3) for Thermal Analysis TGA DTA
- Arc-Shaped Alumina Ceramic Crucible High Temperature Resistant for Engineering Advanced Fine Ceramics
- Engineering Advanced Fine Ceramics Alumina Al2O3 Crucible With Lid Cylindrical Laboratory Crucible
- Engineering Advanced Fine Alumina Al2O3 Ceramic Crucible for Laboratory Muffle Furnace
- Alumina Al2O3 Ceramic Crucible Semicircle Boat with Lid for Engineering Advanced Fine Ceramics
People Also Ask
- Why Use Alumina Crucibles for TGA of Bicyclic Carbonates? Ensure Data Purity & Chemical Inertness
- What are the advantages of selecting an alumina crucible for TGA? Ensure High-Precision Thermal Analysis Data
- What are the advantages of using alumina crucibles for the TGA of modified alkyd resins? Ensure Accurate Results
- What is a crucible material for a furnace? A Guide to Choosing the Right High-Temperature Container
- Why are high-purity alumina (Al2O3) crucibles necessary for liquid lead corrosion tests? Ensure Pure Experimental Data



















