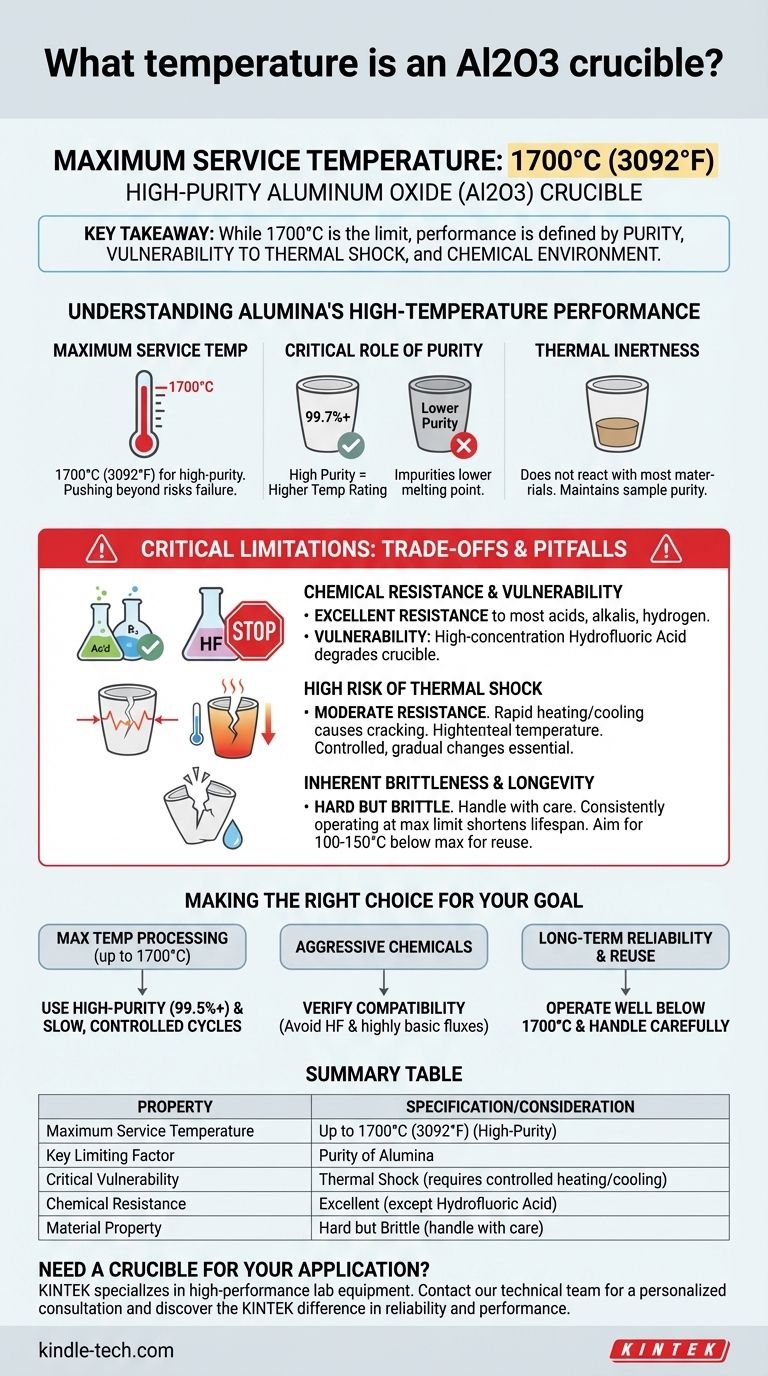
In short, a high-purity aluminum oxide (Al2O3) crucible can be used in applications up to 1700°C (3092°F). This remarkable thermal stability makes it a standard choice for high-temperature work in metallurgy, materials science, and chemistry. However, this temperature rating is not the only factor to consider for successful and safe operation.
The key takeaway is that while 1700°C is the maximum service temperature for alumina, its true performance limit is equally defined by its purity, its vulnerability to thermal shock, and the specific chemical environment it's used in.
Understanding Alumina's High-Temperature Performance
Aluminum oxide, often referred to as alumina, is a ceramic valued for its high melting point and chemical stability. These properties are what allow it to function effectively in extreme environments where metals and other materials would fail.
The Maximum Service Temperature
The 1700°C figure represents the upper limit for continuous use of a high-purity (e.g., 99.7%) alumina crucible. Pushing beyond this temperature risks softening, deformation, and eventual failure of the material.
The Critical Role of Purity
The maximum temperature rating is directly tied to the purity of the Al2O3. Lower-purity crucibles contain other oxides and impurities that can lower the overall melting point and reduce its maximum safe operating temperature.
Thermal Inertness
Alumina's stability means it generally does not react with the materials being heated inside it. This ensures the purity of the sample is maintained, which is critical for scientific experiments and high-quality material production.
Beyond Temperature: Critical Limitations
A material's usefulness is defined as much by its limitations as its strengths. For Al2O3 crucibles, chemical compatibility and physical handling are just as important as the temperature rating.
Chemical Resistance
Alumina exhibits excellent resistance to chemical attacks from most acids and alkaline solutions. It also holds up well against hydrogen and other reducing gases at high temperatures.
A Key Vulnerability
The notable exception to its chemical resistance is high-concentration hydrofluoric acid. This acid will actively attack and degrade the crucible, and contact must be avoided.
Understanding the Trade-offs and Pitfalls
To use an Al2O3 crucible effectively, you must be aware of its operational trade-offs. Misunderstanding these points is a common source of equipment failure.
High Risk of Thermal Shock
Despite its high-temperature stability, alumina has only moderate resistance to thermal shock. Rapid heating or cooling will create internal stresses that can cause the crucible to crack or shatter. Controlled, gradual temperature changes are essential.
Inherent Brittleness
Like most technical ceramics, alumina is a hard but brittle material. It is susceptible to fracture from mechanical shock, such as being dropped or struck by a hard object. Always handle crucibles with care.
Longevity vs. Peak Temperature
Operating a crucible consistently at its absolute maximum temperature limit will significantly shorten its lifespan. For applications requiring frequent use, it is wise to operate at least 100-150°C below the stated maximum.
Making the Right Choice for Your Goal
Your specific application will determine how you should approach using an alumina crucible.
- If your primary focus is maximum temperature processing (up to 1700°C): You must use a high-purity (99.5%+) alumina crucible and implement slow, controlled heating and cooling cycles to prevent thermal shock.
- If your primary focus is working with aggressive chemicals: Verify that your materials do not include hydrofluoric acid or certain highly basic fluxes at extreme temperatures, as these will degrade the crucible.
- If your primary focus is long-term reliability and reuse: Operate well below the 1700°C limit and always handle the crucible carefully to avoid both thermal and mechanical shock.
By respecting both its impressive thermal capacity and its physical limitations, you can effectively utilize alumina for demanding high-temperature applications.
Summary Table:
| Property | Specification / Consideration |
|---|---|
| Maximum Service Temperature | Up to 1700°C (3092°F) for high-purity Al2O3 |
| Key Limiting Factor | Purity of the alumina (e.g., 99.7% vs. lower grades) |
| Critical Vulnerability | Low resistance to thermal shock; requires controlled heating/cooling |
| Chemical Resistance | Excellent, except for hydrofluoric acid and strong fluxes |
| Material Property | Hard but brittle; handle with care to avoid mechanical shock |
Need a crucible that matches your specific high-temperature application?
At KINTEK, we specialize in high-performance lab equipment, including a range of alumina crucibles tailored for different purity requirements and thermal challenges. Our experts can help you select the right crucible to ensure safety, longevity, and accurate results in your metallurgy, materials science, or chemistry work.
Contact our technical team today for a personalized consultation and discover the KINTEK difference in reliability and performance.
Visual Guide
Related Products
- Engineering Advanced Fine Ceramics Alumina Al2O3 Crucible With Lid Cylindrical Laboratory Crucible
- Engineering Advanced Fine Ceramics Alumina Crucibles (Al2O3) for Thermal Analysis TGA DTA
- Alumina Al2O3 Ceramic Crucible Semicircle Boat with Lid for Engineering Advanced Fine Ceramics
- Engineering Advanced Fine Alumina Al2O3 Ceramic Crucible for Laboratory Muffle Furnace
- Arc-Shaped Alumina Ceramic Crucible High Temperature Resistant for Engineering Advanced Fine Ceramics
People Also Ask
- How does an alumina crucible function during NZSP sintering? Optimize Your Solid Electrolyte Performance
- Why is a graphite crucible containing molten bismuth used in LiF–NaF–KF purification? Enhance Melt Purity Efficiently
- Do crucibles break easily? Understanding Thermal Shock and Proper Handling
- What role does a ceramic boat play in the carbonization of aluminum-based metal-organic frameworks? Ensure High Purity
- What is the technical value of using graphite crucibles with graphite paper liners? Optimize Zr3(Al1-xSi)C2 Synthesis
- What is the purpose of using a platinum crucible in LAGP synthesis? Ensure Purity in High-Temperature Reactions
- Do you have to temper your crucible? A Critical Safety Step for Melting Metal
- How are carbon crucibles made? Discover the Engineering Behind High-Performance Crucibles



















