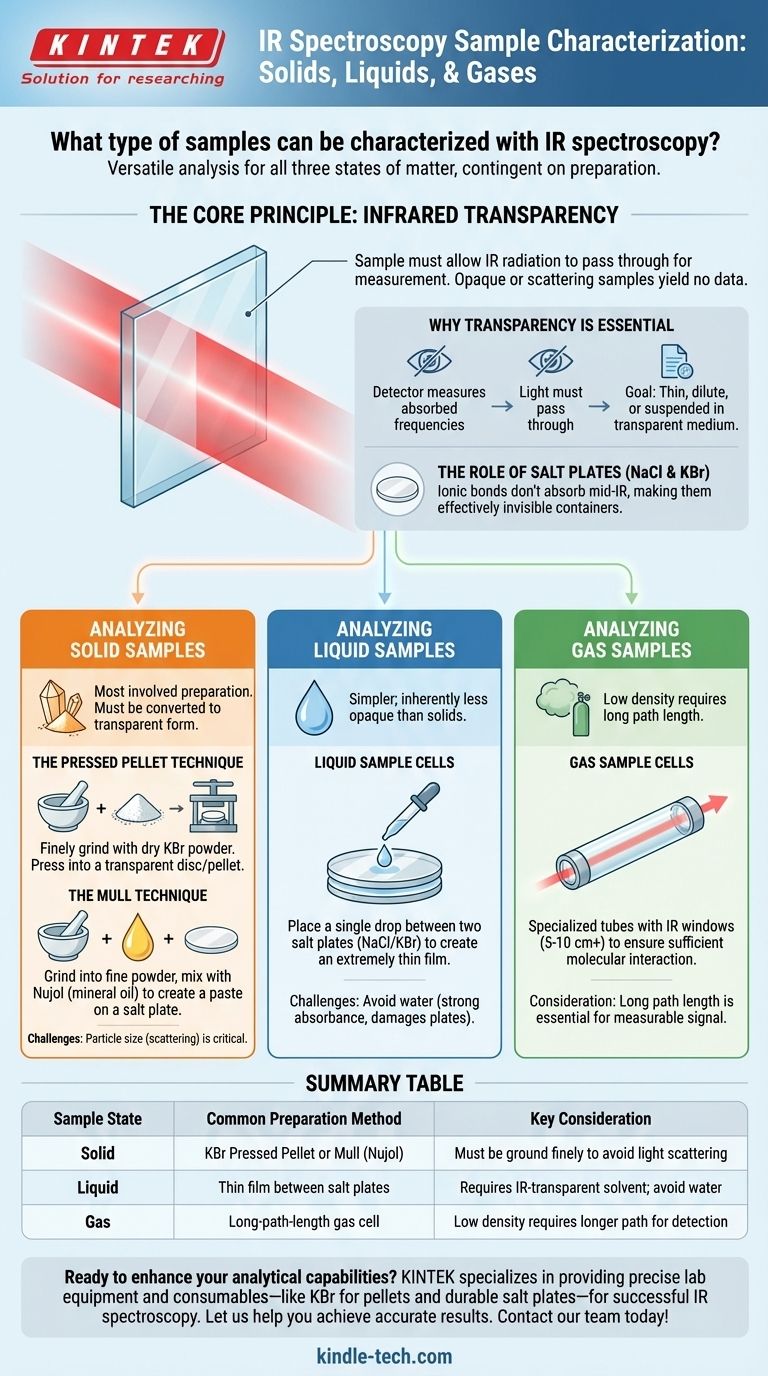
In short, IR spectroscopy is a remarkably versatile technique capable of analyzing samples in all three primary states of matter: solid, liquid, and gas. The key is not the physical state of the sample itself, but how it is prepared for the analysis.
The single most important requirement for any IR spectroscopy sample is infrared transparency. The sample, and any material used to hold or contain it, must allow IR radiation to pass through it for a successful measurement.

The Core Principle: Infrared Transparency
To understand what samples can be used, we must first understand how the technique works. IR spectroscopy measures the specific frequencies of infrared light that are absorbed by a sample's chemical bonds.
Why Transparency Is Essential
For the instrument's detector to measure which frequencies were absorbed, the light must first be able to pass through the sample. If the sample is opaque or scatters the light too much, no meaningful data can be collected.
This principle dictates every aspect of sample preparation. The goal is always to create a sample that is thin enough, dilute enough, or suspended in a medium that is transparent to the IR beam.
The Role of Salt Plates (NaCl & KBr)
You will often see materials like Sodium Chloride (NaCl) and Potassium Bromide (KBr) used to make sample holders, windows, and pellets. This is because their ionic bonds do not absorb light in the mid-infrared region, making them effectively invisible to the spectrometer.
Analyzing Solid Samples
Preparing solid samples is often the most involved process because they must be converted into a form that is transparent to IR radiation.
The Pressed Pellet Technique
The most common method involves finely grinding a small amount of the solid sample with a dry, IR-transparent salt powder, typically Potassium Bromide (KBr). This mixture is then put under immense pressure to form a small, transparent disc or "pellet" that can be placed directly in the spectrometer's beam.
The Mull Technique
An alternative method is to grind the solid into a very fine powder and then mix it with a drop or two of a heavy oil, such as Nujol (a mineral oil). This creates a thick paste, or "mull," which is then smeared onto a salt plate. The oil's own spectrum must be subtracted from the final result.
Other Solid Techniques
For some polymers, a sample can be prepared by dissolving the material in a suitable solvent and evaporating it onto a salt plate, leaving a thin cast film.
Analyzing Liquid and Gas Samples
Liquids and gases are often simpler to prepare because they are inherently less opaque than bulk solids.
Liquid Sample Cells
The most straightforward method for liquids is to place a single drop between two polished salt plates (like NaCl or KBr). The plates are pressed together to create an extremely thin film of the liquid, which is sufficient for analysis.
Gas Sample Cells
Gas samples are analyzed using a specialized gas cell. These are tubes with IR-transparent windows at both ends. Because gases have a very low density and thus absorb very little IR light, these cells must have a very long path length (often 5 to 10 cm or more) to ensure enough molecules interact with the beam to produce a measurable signal.
Understanding the Practical Limitations
While versatile, IR spectroscopy is not without its challenges, most of which relate to sample preparation.
The Challenge of Water
Aqueous (water-based) samples are extremely difficult to analyze. Water itself has very strong, broad IR absorbance bands that can obscure the sample's signals. Furthermore, the most common salt plates (NaCl and KBr) are water-soluble and will be quickly damaged.
Sample Preparation is Key
Poor preparation is the most common source of bad data. For solids, if the particles are not ground finely enough, they will scatter the IR beam rather than absorbing it, leading to a distorted and unusable spectrum.
Concentration and Path Length
The strength of an IR signal is proportional to the concentration of the sample and the distance the light travels through it. This is why pure liquids are run in very thin films, while low-concentration gases require very long path lengths.
Making the Right Choice for Your Sample
Your approach will be dictated entirely by the physical state and properties of the material you need to analyze.
- If your primary focus is a pure solid: The KBr pellet and mull techniques are standard methods for producing high-quality, quantitative data.
- If your primary focus is a pure liquid: A thin film between two salt plates is the fastest and most direct method of analysis.
- If your primary focus is a gas: You will require a specialized long-path-length gas cell to obtain a measurable signal.
- If your sample is dissolved in a solvent: You must ensure the solvent itself is transparent in the IR region of interest and use a liquid cell that is resistant to that solvent.
Ultimately, successful IR analysis depends less on the sample's state and more on correctly preparing it to be transparent to infrared light.
Summary Table:
| Sample State | Common Preparation Method | Key Consideration |
|---|---|---|
| Solid | KBr Pressed Pellet or Mull (Nujol) | Must be ground finely to avoid light scattering |
| Liquid | Thin film between salt plates (NaCl/KBr) | Requires IR-transparent solvent; avoid water |
| Gas | Long-path-length gas cell | Low density requires longer path for detection |
Ready to enhance your analytical capabilities? KINTEK specializes in providing the precise lab equipment and consumables—like KBr for pellet preparation and durable salt plates—you need for successful IR spectroscopy. Our experts understand the critical role of proper sample preparation. Let us help you achieve accurate, reliable results. Contact our team today to discuss your laboratory requirements!
Visual Guide
Related Products
- Single Punch Electric Tablet Press Machine Laboratory Powder Tablet Punching TDP Tablet Press
- Optical Window Glass Substrate Wafer Barium Fluoride BaF2 Substrate Window
- Thin-Layer Spectral Electrolysis Electrochemical Cell
- Heated Hydraulic Press Machine with Heated Plates for Vacuum Box Laboratory Hot Press
- Double Plate Heating Press Mold for Lab
People Also Ask
- How does a sputter coater work? A Guide to Atomic-Level Thin Film Deposition
- Does higher heat capacity mean higher melting point? Unraveling the Critical Difference
- How do rotary vane pumps compare to liquid ring vacuum pumps? Choosing the Right Vacuum Pump for Your Process
- What is a laboratory heater? A Guide to Precision, Safety, and Choosing the Right Type
- What are some ways you can prevent injury when dealing with hot substances and objects? A Proactive Framework for Thermal Safety
- How does a centrifuge separate particles? Master the Science of High-Speed Separation
- What is the voltage for arcing? It's Not a Single Number—It's About Electric Field Strength
- When was magnetron sputtering invented? The 1970s Breakthrough That Revolutionized Thin-Film Coating















