Graphite Furnace Atomic Absorption Spectroscopy (GFAAS) is more sensitive than Flame AAS (FAAS) because it confines a discrete sample of atoms in the instrument's light path for a significantly longer time. FAAS continuously aspirates a sample through a flame, giving atoms mere milliseconds to absorb light. In contrast, GFAAS heats a sample inside a small graphite tube, creating a concentrated cloud of atoms that remains in the light path for several seconds, dramatically increasing the probability of light absorption and producing a much stronger signal.
The core difference is not that the graphite furnace creates more atoms, but that it holds them in the measurement zone with exceptional efficiency. This extended atom residence time is the fundamental reason GFAAS can achieve detection limits 100 to 1,000 times lower than FAAS.

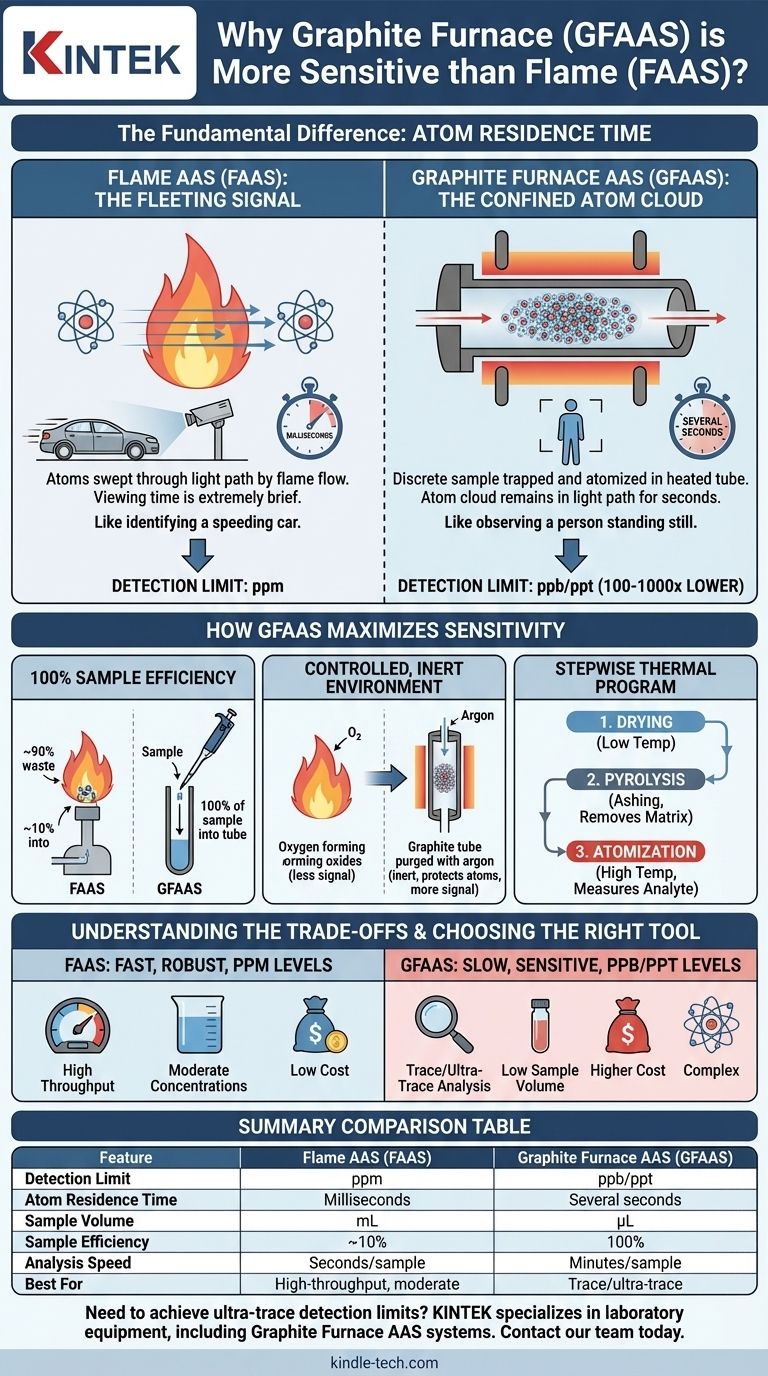
The Fundamental Difference: Atom Residence Time
The sensitivity of any atomic absorption technique is directly related to how long the target atoms are present in the path of the light beam. This single factor, residence time, is the primary driver of the performance gap between Flame and Graphite Furnace AAS.
The Fleeting Signal of the Flame (FAAS)
In Flame AAS, a liquid sample is continuously drawn into a nebulizer and sprayed into a high-temperature flame.
The atoms are created and swept through the light path by the high-velocity flow of the flame gases. This entire process is incredibly fast, with any given atom residing in the light path for only a few milliseconds.
This is analogous to trying to identify a person in a car speeding past you on a highway; the viewing time is extremely brief.
The Confined Atom Cloud of the Furnace (GFAAS)
GFAAS operates on a completely different principle. A small, precise volume of the sample (typically in microliters) is placed directly into a graphite tube.
The tube is then heated electrothermally in a programmed sequence. When the final, high-temperature atomization step occurs, a dense cloud of atoms is generated and physically trapped within the confines of the tube.
This cloud remains in the light path for several seconds, orders of magnitude longer than in FAAS. This is like observing a person standing still; you have ample time to make a positive identification.
How GFAAS Maximizes Sensitivity
Beyond residence time, several other factors inherent to the graphite furnace design contribute to its superior performance for trace analysis.
100% Sample Efficiency
In FAAS, the vast majority of the sample aspirated—often over 90%—goes directly to waste and never reaches the flame. The nebulizer is only efficient at creating a fine aerosol from a small fraction of the liquid.
GFAAS, by contrast, uses the entire discrete sample volume placed into the tube. This absolute efficiency ensures that all the analyte present in the sample contributes to the final signal.
Controlled, Inert Environment
The graphite tube is continuously purged with an inert gas, such as argon. This prevents the hot, reactive atoms from forming oxides, which do not absorb light at the correct wavelength.
A flame is an open, oxidative environment. A significant portion of the atoms can be lost to oxidation, reducing the measurable signal. The inert GFAAS environment protects the atomic population.
Stepwise Thermal Program
GFAAS uses a multi-step heating program that provides a powerful means of sample cleanup before the measurement step.
- Drying: The solvent is gently evaporated at a low temperature.
- Pyrolysis (Ashing): The temperature is increased to thermally decompose the sample matrix (salts, organic matter) and vent it away, leaving the more thermally stable analyte behind.
- Atomization: The temperature is rapidly ramped to thousands of degrees Celsius, vaporizing the analyte into a dense atom cloud for measurement.
This programmable cleanup is impossible in a flame, where the sample, solvent, and matrix are all introduced simultaneously.
Understanding the Trade-offs
The superior sensitivity of GFAAS comes with significant practical trade-offs. It is not always the better choice.
Speed and Throughput
FAAS is exceptionally fast. Once calibrated, a sample can be analyzed in a matter of seconds. This makes it ideal for high-throughput labs analyzing many samples.
GFAAS is inherently slow. A single sample run, including the drying, pyrolysis, and atomization steps, takes several minutes.
Cost and Complexity
FAAS instruments are generally less expensive, simpler to operate, and more robust. The primary consumable is the gas for the flame.
GFAAS systems are more expensive and complex. The graphite tubes are a significant consumable cost, as they have a limited lifetime of a few hundred firings before they must be replaced. Method development is also more demanding.
Matrix Interferences
While the pyrolysis step helps, GFAAS can be very susceptible to chemical and background interferences from complex sample matrices. This often requires advanced techniques like matrix modifiers or Zeeman background correction.
FAAS is often considered more "rugged" and forgiving for samples with high dissolved solids, though it has its own set of interferences.
Making the Right Choice for Your Analysis
Choosing between these two powerful techniques depends entirely on your analytical goals.
- If your primary focus is trace or ultra-trace analysis (ppb/ppt levels): The superior sensitivity and low sample volume requirements make GFAAS the definitive choice.
- If your primary focus is high sample throughput and moderate concentrations (ppm levels): The speed, robustness, and lower operating cost of FAAS make it the more efficient and practical option.
- If you are analyzing samples with high dissolved solids for major components: FAAS is almost always the more reliable and straightforward tool for the job.
Ultimately, understanding these core principles allows you to select the right tool for your specific analytical challenge.
Summary Table:
| Feature | Flame AAS (FAAS) | Graphite Furnace AAS (GFAAS) |
|---|---|---|
| Detection Limit | ppm (parts per million) | ppb/ppt (parts per billion/trillion) |
| Atom Residence Time | Milliseconds | Several seconds |
| Sample Volume | mL | µL (microliters) |
| Sample Efficiency | ~10% | 100% |
| Analysis Speed | Seconds per sample | Minutes per sample |
| Best For | High-throughput, moderate concentrations | Trace/ultra-trace analysis |
Need to achieve ultra-trace detection limits in your lab? KINTEK specializes in laboratory equipment and consumables, including Graphite Furnace AAS systems. Our experts can help you select the right instrument to enhance your analytical capabilities, ensuring precise, reliable results for even the most challenging samples. Contact our team today to discuss your specific laboratory needs and how we can support your research and quality control goals.
Visual Guide
Related Products
- Graphite Vacuum Continuous Graphitization Furnace
- Vertical High Temperature Graphite Vacuum Graphitization Furnace
- Multi-zone Laboratory Tube Furnace
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Vacuum Hot Press Furnace Machine for Lamination and Heating
People Also Ask
- How is synthetic graphite manufactured? A Deep Dive into the High-Temperature Process
- What is the high temperature graphite material? The Ultimate Solution for Extreme Heat Applications
- What is the function of the graphite furnace? Achieve Extreme Heat for Analysis & Materials Processing
- What is special about graphite? Unlocking Its Unique Properties for Extreme Applications
- Can graphite conduct electricity and heat? The Unique Properties of a Non-Metal Conductor
- What are the industrial uses of graphite? Leverage Its Unique Properties for Demanding Applications
- How does a graphite furnace work? Achieve Extreme Temperatures in a Pure Environment
- What are the interferences of graphite furnace? Overcome Matrix & Spectral Issues for Accurate GFAAS



















