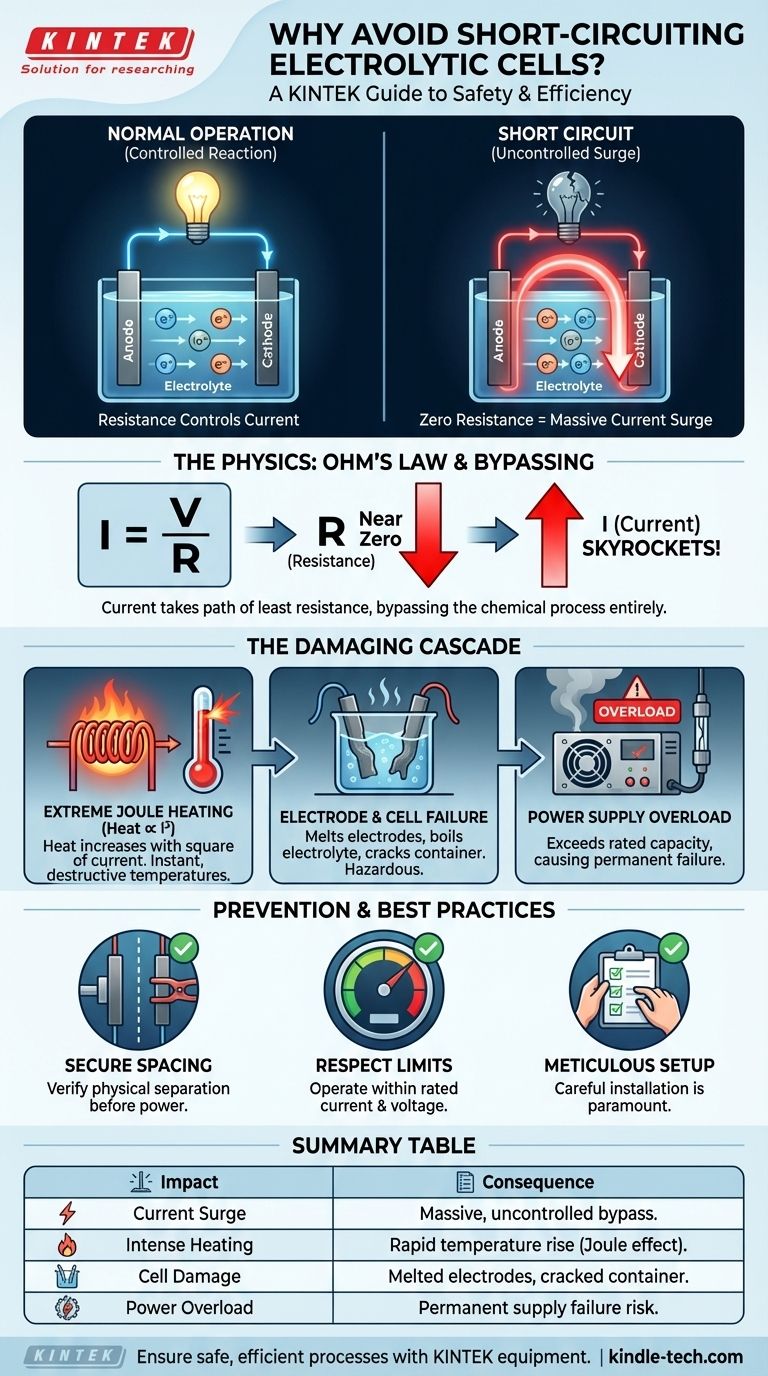
To put it simply, avoiding a short circuit between the electrodes of an electrolytic cell is critical because it causes an uncontrolled and massive flow of electrical current. This current surge bypasses the intended chemical reaction entirely, generating extreme heat that can destroy the electrodes, damage the cell itself, and overload your power supply.
A short circuit fundamentally changes the purpose of your cell. Instead of driving a controlled chemical process, it becomes a simple, low-resistance path for electricity, converting electrical energy directly into destructive heat.

The Physics Behind a Short Circuit
To grasp the danger, you need to understand what happens to the electrical current when its intended path is compromised.
What is a Short Circuit?
A short circuit is a low-resistance connection between two points in an electrical circuit that are supposed to have different voltages. In an electrolytic cell, this means the anode and cathode physically touching or being connected by an unintended conductor.
The Role of Ohm's Law
Ohm's Law (Current = Voltage / Resistance) governs the flow of electricity. In normal operation, the electrolyte provides a specific resistance, which carefully controls the amount of current flowing and drives the desired electrochemical reaction.
When the electrodes touch, the resistance in the circuit drops to nearly zero. According to the formula, dividing the voltage by a near-zero resistance causes the current to skyrocket toward an extremely high value, limited only by what the power supply can deliver.
Bypassing the Electrolyte
Electricity always follows the path of least resistance. The short circuit provides a much easier path for current than moving through the electrolyte. As a result, the electrochemical process stops completely, and all the system's power is shunted through the short.
The Cascade of Damaging Effects
This uncontrolled surge of current sets off a chain reaction that quickly leads to system failure and potential danger.
Extreme Current and Overheating
The primary consequence of massive current is intense heat generation, an effect known as Joule heating. The heat produced is proportional to the square of the current, meaning a 10x increase in current results in a 100x increase in heat. This can instantly raise temperatures to destructive levels.
Damage to Electrodes and the Cell
This extreme heat can easily melt or warp the electrodes, rendering them useless. It can also boil the electrolyte, crack the cell container, and damage any surrounding components, potentially releasing hazardous materials.
Overloading the Power Supply
The power supply is forced to try and meet this near-infinite demand for current. This will almost certainly exceed its rated capacity, causing it to overheat, blow a fuse, trip a circuit breaker, or fail permanently.
Common Pitfalls to Avoid
Understanding the difference between controlled resistance and a short is key to successful operation.
Misinterpreting Resistance
In a properly functioning cell, the electrolyte's resistance is a necessary feature, not a flaw. It dictates the rate of your reaction. Attempting to reduce this resistance by moving electrodes too close together risks an accidental short circuit.
Neglecting Physical Spacing
The most common cause of a short is inadequate separation between the electrodes during installation. They must be securely fixed in place to prevent them from moving and touching once the experiment is underway.
Exceeding Rated Limits
As noted in equipment specifications, you must never exceed the rated current and voltage for the cell. A short circuit is the most extreme example of this, but even pushing the limits during normal operation can cause cumulative damage over time.
How to Apply This to Your Project
To ensure a safe and effective outcome, your approach should be guided by your primary objective.
- If your primary focus is safety: Always verify complete physical and electrical separation between the electrodes before applying power.
- If your primary focus is equipment longevity: Operate well within the specified current and voltage limits to prevent stress on the cell and power supply.
- If your primary focus is experimental success: Recognize that a short circuit yields no useful data and only serves to destroy your setup, so meticulous installation is paramount.
By respecting the fundamental principles of your circuit, you protect your investment and ensure the integrity of your electrochemical process.
Summary Table:
| Consequence of a Short Circuit | Key Impact |
|---|---|
| Extreme Current Surge | Massive, uncontrolled current bypasses the chemical reaction. |
| Intense Joule Heating | Heat increases with the square of the current, causing rapid temperature rise. |
| Electrode & Cell Damage | Electrodes can melt or warp; the cell container can crack. |
| Power Supply Overload | Exceeds rated capacity, risking permanent damage or failure. |
Ensure your lab's electrochemical processes are safe and efficient with the right equipment. KINTEK specializes in high-quality lab equipment and consumables, providing reliable solutions for your laboratory needs. Avoid costly downtime and damaged experiments—contact our experts today to find the perfect equipment for your application!
Visual Guide
Related Products
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Optical Water Bath Electrolytic Electrochemical Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
People Also Ask
- What are the typical volumes and aperture configurations for a double-layer water-bath electrolytic cell? Optimize Your Electrochemical Setup
- What are the standard aperture specifications of the electrolytic cell? Key Sizes for Your Electrochemical Setup
- What are the procedures for after using a double-layer water-bath electrolytic cell? Ensure Equipment Longevity and Data Accuracy
- When is professional repair required for a double-layer water-bath electrolytic cell? Protect Your Lab's Precision and Safety
- What is the overall structure of the H-type double-layer optical water bath electrolytic cell? Precision Design for Controlled Experiments



















