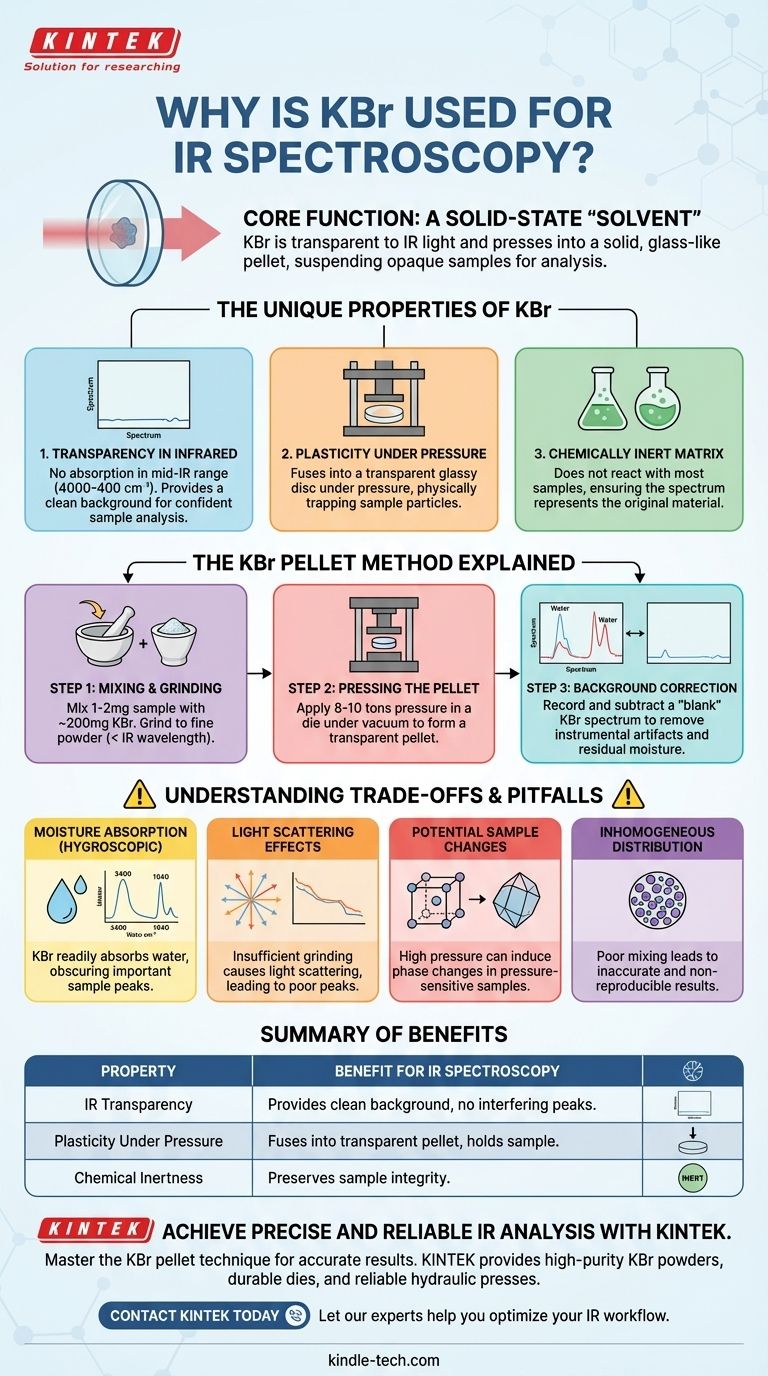
In short, Potassium Bromide (KBr) is used for IR spectroscopy because it is transparent to infrared light and can be pressed into a solid, glass-like pellet. This allows a solid sample, which is normally opaque, to be held in the spectrometer's beam path in a way that allows the light to pass through it for analysis.
The core function of KBr is to act as a solid-state "solvent." It creates an optically clear, non-interfering matrix that suspends the sample, making it possible to measure the infrared spectrum of a solid material that cannot be easily dissolved or melted.

The Unique Properties of KBr
Transparency in the Infrared Region
The most critical property of KBr is its lack of absorption in the mid-infrared range (typically 4000 to 400 cm⁻¹).
Because KBr itself does not absorb this light, it provides a clean background. Any absorption peaks seen in the final spectrum can be confidently attributed to the sample, not the KBr matrix.
Plasticity Under Pressure
KBr is an alkali halide, a class of salts that exhibit plastic deformation. When subjected to several tons of pressure, the crystalline KBr powder flows and fuses into a single, transparent, glassy disc.
This process physically traps the finely ground sample particles within the newly formed KBr sheet, holding them in place for analysis.
A Chemically Inert Matrix
For most organic and many inorganic compounds, KBr is chemically inert. It does not react with the sample during preparation or measurement.
This ensures that the resulting spectrum is representative of the original sample, not some new compound formed by a reaction with the matrix.
The KBr Pellet Method Explained
Step 1: Mixing and Grinding
A very small amount of the solid sample (typically 1-2 mg) is added to a much larger amount of high-purity, dry KBr powder (around 100-200 mg).
This mixture is then ground extensively in an agate mortar and pestle. The goal is to reduce the particle size of the sample to less than the wavelength of the IR light to minimize light scattering.
Step 2: Pressing the Pellet
The finely ground powder is placed into a specialized die. The die is often placed under a vacuum to remove trapped air and, more importantly, atmospheric moisture.
A hydraulic press is then used to apply immense pressure (8-10 tons) to the die, compressing the powder and causing the KBr to fuse into a transparent pellet.
Step 3: Background Correction
Before or after running the sample, a "blank" spectrum is often recorded using a pellet made of pure KBr. This allows the instrument's software to subtract any minor absorptions from residual moisture or instrumental artifacts, resulting in a cleaner sample spectrum.
Understanding the Trade-offs and Pitfalls
The Problem of Moisture
KBr is hygroscopic, meaning it readily absorbs water from the atmosphere. This is the single most common problem when using the KBr method.
Water has very strong and broad IR absorption bands (a wide peak around 3400 cm⁻¹ and another near 1640 cm⁻¹). If your KBr is "wet," these large water peaks can completely obscure important peaks from your actual sample. Using desiccated KBr and minimizing exposure to air is critical.
Light Scattering Effects
If the sample is not ground finely enough, its particles will scatter the infrared light instead of simply absorbing it. This leads to a distorted spectrum with a sloping baseline and poorly defined peaks (an issue known as the Christiansen effect).
Potential for Sample Changes
The high pressure used to form the pellet can occasionally induce a phase change in the sample's crystal structure (polymorphism). This means the spectrum you measure might not be of the exact same crystalline form you started with.
Inhomogeneous Distribution
If the sample is not mixed thoroughly with the KBr, its concentration will not be uniform throughout the pellet. This can lead to inaccurate and non-reproducible results, which is a significant problem for quantitative analysis.
Making the Right Choice for Your Analysis
Briefly preparing for the pellet-making process will ensure you get a high-quality spectrum that accurately reflects your sample.
- If your primary focus is simple compound identification: Prioritize using bone-dry KBr and grinding the sample thoroughly to get clean, well-defined peaks free from water and scattering artifacts.
- If your primary focus is quantitative measurement: Meticulous weighing of the sample and KBr and ensuring a perfectly homogeneous mixture is non-negotiable for achieving reproducible results.
- If you are analyzing a pressure-sensitive material: Consider an alternative solid sampling method, such as a Nujol mull, which involves grinding the sample in mineral oil and does not require high pressure.
Ultimately, mastering the KBr pellet technique provides a powerful and reliable window into the molecular structure of a vast range of solid materials.
Summary Table:
| Property | Benefit for IR Spectroscopy |
|---|---|
| IR Transparency | Provides a clean background with no interfering absorption peaks. |
| Plasticity Under Pressure | Fuses into a transparent pellet that holds the sample. |
| Chemical Inertness | Does not react with most samples, preserving their integrity. |
Achieve precise and reliable IR analysis with KINTEK.
Mastering the KBr pellet technique is essential for accurate solid sample identification. KINTEK specializes in providing the high-purity KBr powders, durable pellet dies, and reliable hydraulic presses you need to ensure your sample preparation is consistent and contamination-free.
Our lab equipment and consumables are designed to help you overcome common challenges like moisture absorption and light scattering, delivering clear, interpretable spectra every time.
Contact KINTEK today to discuss your laboratory's specific needs and let our experts help you optimize your IR spectroscopy workflow.
Visual Guide
Related Products
- XRF & KBR steel ring lab Powder Pellet Pressing Mold for FTIR
- XRF & KBR plastic ring lab Powder Pellet Pressing Mold for FTIR
- Optical Window Glass Substrate Wafer Barium Fluoride BaF2 Substrate Window
- Laboratory Hydraulic Press Lab Pellet Press for Button Battery
- Optical Ultra-Clear Glass Sheet for Laboratory K9 B270 BK7
People Also Ask
- Why is it important to match the freezer temperature to storage recommendations? Optimize Food Safety & Energy Use
- What is sintering of clay materials? The Science of Turning Clay into Durable Ceramics
- What apparatus is used for drying specimens? Select the Right Tool to Preserve Your Sample Integrity
- What role does a high-shear dispersion emulsifier play in ionic liquid-based Pickering emulsions? Achieve Lab Precision
- What are the properties of refrigerant fluids used in Ultra Freezers? Achieving Reliable -86°C Performance
- What is the long-term stability of viral analytes in plasma stored at -70°C? Proven for Decades of Research
- Which of the following are methods used to deposit thin films? A Guide to PVD, CVD & More
- What does CIP stand for Crip? Understanding the Meaning of Crip In Peace

















