Historical Discoveries in Electrochemistry
Galvani's Frog Experiment
In 1791, the Italian scientist Luigi Galvani conducted a groundbreaking experiment that would forever link biology and electrochemistry. During the dissection of a frog's leg, Galvani noticed that the leg twitched when it came into contact with two different metals. This seemingly simple observation led to a profound realization: biological tissues could generate and respond to electrical signals.
This discovery was not merely an academic curiosity; it had practical implications as well. The phenomenon observed by Galvani was later named "galvanic action," and it laid the foundation for the development of devices such as the galvanometer, an instrument used to measure small electric currents. Furthermore, the process of galvanization—the coating of metals to prevent corrosion—was named in his honor, underscoring the lasting impact of his work.
Galvani's experiment was a pivotal moment in the history of science, bridging the gap between life sciences and physical sciences. It demonstrated that biological systems could be understood through the lens of electrochemical principles, paving the way for future advancements in both fields.
Volta's Invention of the Voltaic Pile
In 1799, Alessandro Volta, an Italian physicist, made a groundbreaking discovery that laid the foundation for modern electrochemical systems. He invented the first chemical power source, known as the Voltaic pile, which was essentially a stack of copper and zinc discs separated by a moistened cloth or cardboard soaked in brine. This simple yet ingenious device could generate a steady electric current, marking the dawn of practical electricity generation.
The significance of Volta's invention was quickly realized, and it spurred further research into the potential applications of this newfound energy source. Just a year later, in 1800, William Nicholson and Anthony Carlisle used a Voltaic pile to conduct the first successful electrolysis of water. During this experiment, they observed the precipitation of gases, specifically hydrogen and oxygen, which confirmed the decomposition of water molecules into their constituent elements under the influence of an electric current.
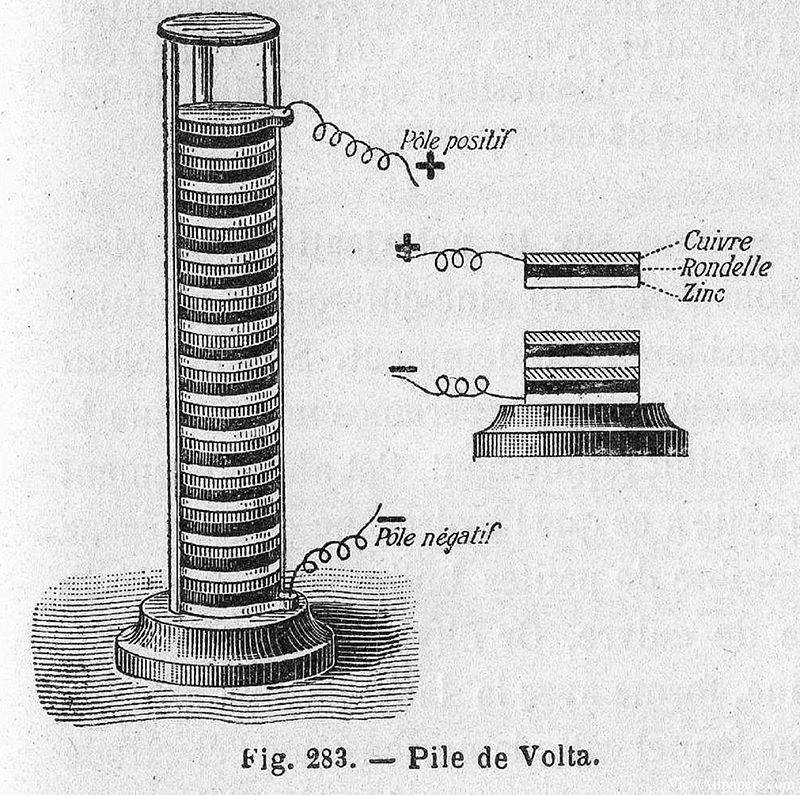
This discovery not only validated the concept of electrolysis but also demonstrated the practical utility of Volta's pile in scientific experimentation. The ability to decompose compounds into their elemental parts using electricity opened up new avenues for research in chemistry and physics, paving the way for future advancements in electrochemical theory and applications.
Electrolysis Successes
In 1803, Humphry Davy achieved a groundbreaking success in the field of electrochemistry by successfully isolating potassium and sodium metals through the process of electrolysis. This monumental discovery not only demonstrated the power of electrochemical methods but also paved the way for the production of active metal monomers, which are essential in various industrial applications.
Davy's experiments involved the use of a Voltaic pile, the first chemical power source invented by Alessandro Volta. By passing an electric current through molten salts, Davy was able to decompose these compounds and obtain pure metals. This technique marked a significant advancement in the extraction of reactive metals, which were previously difficult to isolate using traditional chemical methods.
The implications of Davy's work extended beyond the laboratory. His success in electrolysis laid the foundation for future research in electrochemical processes, leading to the development of more efficient methods for metal extraction and purification. This discovery also underscored the potential of electrochemical techniques in various industrial sectors, from metallurgy to chemical manufacturing.
Moreover, Davy's findings contributed to the broader understanding of electrochemical principles, setting the stage for subsequent theoretical advancements such as Faraday's Law of Electrolysis and Nernst's Equation. These theoretical frameworks, in turn, enabled more precise quantitative studies of electrochemical phenomena, further advancing the field.
In summary, Davy's successful electrolysis of potassium and sodium in 1803 was a pivotal moment in the history of electrochemistry, demonstrating the practical applications of electrochemical methods and laying the groundwork for future innovations in the field.
Theoretical Foundations of Electrochemistry
Faraday's Law of Electrolysis
In 1834, Michael Faraday, a pioneering physicist and chemist, formulated the law of electrolysis, a cornerstone in the field of electrochemistry. This law provided a quantitative framework for understanding and analyzing electrochemical phenomena, significantly advancing the theoretical underpinnings of the discipline. Faraday's work was instrumental in bridging the gap between empirical observations and theoretical models, laying the groundwork for future discoveries and applications in electrochemistry.
Faraday's law of electrolysis consists of two primary principles:
- First Law: The mass of a substance deposited or liberated at an electrode during electrolysis is directly proportional to the quantity of electricity passed through the electrolyte.
- Second Law: The mass of a substance deposited or liberated at an electrode during electrolysis is directly proportional to the chemical equivalent weight of the substance.
These laws enabled scientists to precisely measure the amount of material transformed during an electrochemical reaction, facilitating the development of standardized procedures and techniques in electrochemistry. By quantifying the relationship between electrical current, time, and the resulting chemical changes, Faraday's laws provided a robust method for studying and predicting electrochemical processes.
The impact of Faraday's work extended beyond mere quantification. His laws fostered a deeper understanding of the mechanisms underlying electrolysis, which in turn led to advancements in various practical applications. These applications ranged from industrial processes like metal refining and electroplating to the development of batteries and fuel cells, which are integral to modern energy systems.
Moreover, Faraday's contributions set the stage for subsequent theoretical developments in electrochemistry. His laws were foundational to the work of later scientists such as Walther Nernst, who derived the Nernst equation, and Julius Tafel, who formulated Tafel's equation. These advancements further refined the understanding of electrode potentials, reaction kinetics, and the behavior of ions in solution, all of which are critical aspects of modern electrochemical research.
In summary, Faraday's law of electrolysis not only revolutionized the quantitative study of electrochemical phenomena but also laid a solid theoretical foundation for the burgeoning field of electrochemistry. His work continues to influence and inspire contemporary research and applications, underscoring the enduring significance of his contributions to science.
Nernst's Equation
In 1889, the German chemist Walther Nernst made a groundbreaking contribution to the field of electrochemistry by deriving a mathematical relationship that linked electrode potential to the concentration of substances involved in the electrochemical reaction. This relationship, now known as the Nernst equation, has become a cornerstone in the study of electrochemical systems.
The Nernst equation provides a quantitative means to predict the potential of an electrochemical cell under non-standard conditions, which is crucial for understanding and controlling electrochemical processes. It is expressed as:
[ E = E^\circ - \frac{RT}{nF} \ln Q ]
where:
- ( E ) is the electrode potential.
- ( E^\circ ) is the standard electrode potential.
- ( R ) is the universal gas constant.
- ( T ) is the temperature in Kelvin.
- ( n ) is the number of moles of electrons transferred in the reaction.
- ( F ) is the Faraday constant.
- ( Q ) is the reaction quotient.
The equation demonstrates how changes in concentration can influence the potential of an electrode, which is vital for applications ranging from battery design to industrial electrolysis processes. By allowing scientists to predict and manipulate electrode potentials, the Nernst equation has significantly advanced our ability to engineer and optimize electrochemical systems.
This discovery was not just a theoretical triumph but also a practical one, as it provided a tool for scientists and engineers to better understand and control the behavior of electrochemical cells under various conditions. The Nernst equation remains an indispensable part of electrochemical theory and practice, highlighting the profound impact of Nernst's work on the field.
Tafel's Equation
In 1905, Julius Tafel introduced a groundbreaking empirical formula that established a direct relationship between current density and hydrogen overpotential. This equation, known as Tafel's Equation, played a pivotal role in advancing our understanding of electrochemical kinetics. Tafel's work was instrumental in elucidating the mechanisms underlying the rate of electrochemical reactions, particularly those involving hydrogen evolution.
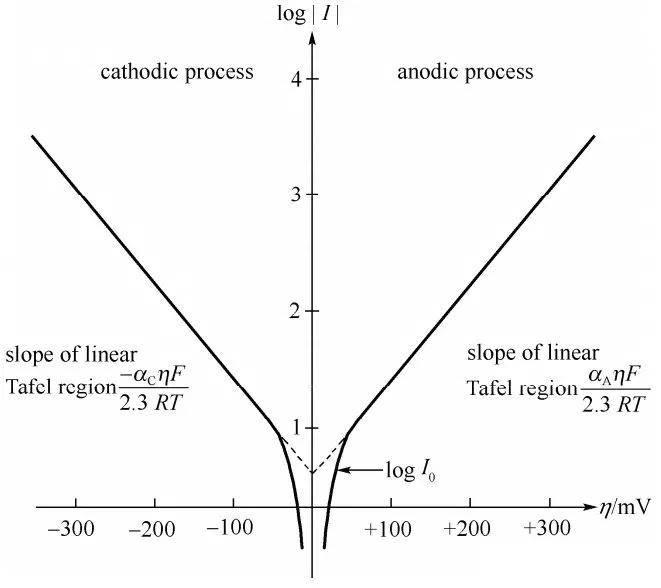
Tafel's Equation is typically expressed as:
$$ \eta = a + b \log(i) $$
where:
- (\eta) is the overpotential,
- (i) is the current density,
- (a) and (b) are constants specific to the electrode material and reaction conditions.
The significance of Tafel's Equation lies in its ability to quantitatively describe the rate of electrochemical processes, which is crucial for optimizing electrode materials and reaction conditions. This empirical law has been extensively validated and applied in various electrochemical systems, from basic research to industrial applications.
| Parameter | Description |
|---|---|
| (\eta) | Overpotential, the extra voltage required beyond the equilibrium potential |
| (i) | Current density, the current per unit area of the electrode surface |
| (a) | Constant related to the exchange current density and the symmetry factor |
| (b) | Tafel slope, related to the charge transfer coefficient |
Tafel's pioneering work laid the foundation for subsequent research in electrochemical kinetics, influencing the development of more sophisticated theories and techniques. His equation remains a cornerstone in the field, highlighting the intricate interplay between electrical and chemical processes in electrochemistry.
Electrochemical Kinetics and Techniques
From 1940 onwards, the field of interfacial electrochemistry experienced significant advancements, particularly in the understanding of the structure of the double layer and the kinetics of hydrogen precipitation. These developments laid the groundwork for more sophisticated theories and techniques in electrochemical kinetics. One of the most notable contributions during this period was the establishment of Marcus's microscopic theory of electron transfer. This theory provided a detailed framework for understanding how electrons move across interfaces, which is crucial for many electrochemical processes.
The advancements in electrochemical kinetics were not limited to theoretical constructs. Experimental techniques also evolved, enabling researchers to probe the behavior of electrons and ions at interfaces with greater precision. For instance, the development of new spectroscopic methods allowed for the real-time observation of chemical reactions occurring at electrode surfaces. These techniques have been instrumental in validating and refining theoretical models, such as Marcus's theory, by providing empirical data that can be directly compared with theoretical predictions.
Moreover, the interplay between theoretical and experimental advancements has led to the development of more efficient electrochemical systems. For example, the understanding of hydrogen precipitation kinetics has been applied in the design of better fuel cells and batteries, which are essential for modern energy storage and conversion technologies. These systems benefit from the improved kinetics, leading to higher efficiency and longer operational lifetimes.
In summary, the period from 1940 onwards marked a transformative era in electrochemical kinetics and techniques. The synergy between theoretical insights and experimental capabilities has not only deepened our understanding of fundamental processes but also paved the way for practical applications in energy and materials science.
In Situ Electrochemical Techniques
Since their inception in the 1970s, in situ electrochemical techniques have revolutionized the exploration of electrochemical mechanisms. These techniques allow researchers to observe and analyze electrochemical processes directly within their operational environments, providing unparalleled insights into the dynamics of reactions at the electrode-electrolyte interface.
One of the most significant advancements in this field is the integration of electrochemical in situ X-ray absorption spectroscopy (XAS). This method enables the real-time monitoring of changes in the electronic structure and oxidation states of materials during electrochemical reactions. By combining XAS with electrochemical techniques, scientists can correlate structural transformations with electrochemical performance, thereby deepening our understanding of catalytic processes and electrode materials.
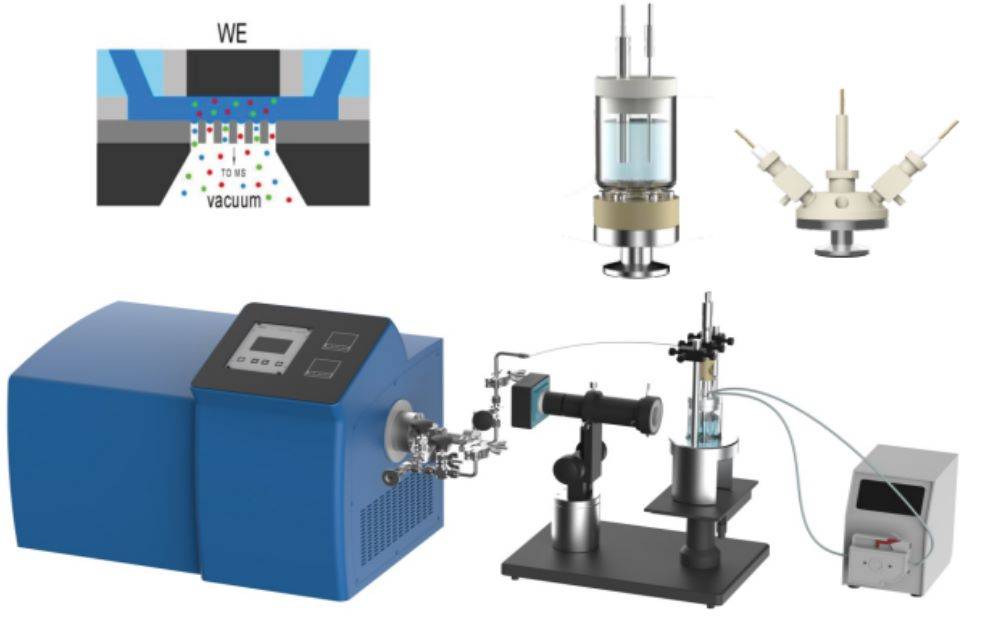
In addition to XAS, other in situ techniques such as scanning electrochemical microscopy (SECM) and in situ Raman spectroscopy have also gained prominence. SECM allows for the spatial resolution of electrochemical processes at the micrometer scale, while in situ Raman spectroscopy provides detailed vibrational information about the species involved in the reactions.
The versatility and precision of in situ electrochemical techniques have not only enhanced fundamental research but also accelerated the development of practical applications. For instance, these techniques are instrumental in optimizing the performance of batteries, fuel cells, and electrocatalysts. By providing real-time data on reaction intermediates and surface modifications, in situ methods help in designing more efficient and durable electrochemical devices.
Moreover, the continuous evolution of these techniques is broadening their applicability. Advances in instrumentation and data analysis methods are enabling higher resolution, faster data acquisition, and more accurate interpretation of results. This progress is paving the way for the next generation of electrochemical research, where in situ techniques will play a pivotal role in unraveling the complexities of electrochemical systems.
Modern Directions in Electrochemistry
Microscopic Electrochemical Mechanisms
The exploration of electrochemical processes at the atomic and molecular scales is a focal point of contemporary research. This field primarily employs in situ and non-in situ techniques to dissect the intricate mechanisms governing these processes. These techniques allow researchers to observe and analyze electrochemical reactions as they occur, providing unprecedented insights into the dynamics at play.
One of the key advancements in this domain is the use of electrochemical in situ X-ray absorption spectroscopy (XAS). This method enables the real-time monitoring of changes in the electronic structure and oxidation states of materials during electrochemical reactions. For instance, XAS can reveal how the surface of a catalyst changes during the catalysis of a specific reaction, offering clues to optimizing the catalyst for better performance.
Additionally, scanning tunneling microscopy (STM) and atomic force microscopy (AFM) have been instrumental in visualizing the surface morphology and electronic properties of electrodes at the atomic level. These techniques have been crucial in understanding how surface defects, adsorbates, and other microscopic features influence the electrochemical behavior of materials.
To construct a comprehensive and rational reaction mechanism, researchers often combine these microscopic observations with theoretical models. For example, density functional theory (DFT) calculations can predict the energy landscapes of potential reaction pathways, while molecular dynamics (MD) simulations can model the time-dependent behavior of ions and molecules in the electrolyte.
The integration of experimental and theoretical approaches has led to significant breakthroughs in the field. For example, the rational design of electrocatalysts for water splitting and CO₂ reduction has been guided by insights gained from these combined efforts. Such advancements not only enhance our fundamental understanding of electrochemical processes but also pave the way for the development of more efficient and sustainable energy technologies.
In summary, the use of advanced in situ and non-in situ techniques, coupled with theoretical modeling, has revolutionized the study of electrochemical mechanisms at the microscopic level. This interdisciplinary approach continues to drive innovation in both academic research and industrial applications.
Advancements in Electrochemical Testing
Traditional electrochemical methods are undergoing significant transformations to meet contemporary demands. These advancements are driven by the need to enhance monitoring sensitivity, adapt to extreme environmental conditions, and integrate sophisticated mathematical treatments. The evolution of electrochemical testing techniques is not merely an incremental improvement but a paradigm shift that bridges the gap between theoretical understanding and practical application.
One of the key areas of development is the integration of advanced mathematical models. These models allow for more precise predictions and interpretations of electrochemical phenomena, particularly in complex systems where traditional methods fall short. For instance, the incorporation of machine learning algorithms has enabled real-time analysis and optimization of electrochemical processes, significantly reducing the time and resources required for experimentation.

Moreover, the adaptability of these methods to extreme conditions has opened new avenues for research and application. Whether it's the high temperatures of industrial processes or the low temperatures of space exploration, modern electrochemical techniques are being tailored to perform reliably and efficiently. This adaptability is crucial for industries ranging from energy production to materials science, where conditions can vary widely.
In addition to mathematical advancements, the hardware components of electrochemical testing have also seen remarkable improvements. High-precision sensors and automated systems are now commonplace, allowing for continuous monitoring and data collection. These systems are not only more accurate but also more user-friendly, making advanced electrochemical testing accessible to a broader range of researchers and industries.
The synergy between these advancements in mathematical modeling, environmental adaptability, and hardware improvements has led to a new era in electrochemical testing. This era is characterized by greater precision, efficiency, and applicability, paving the way for breakthroughs in both fundamental research and practical applications.
Cross-Disciplinary Applications
Electrochemistry's versatility is evident in its wide-ranging applications across various scientific and industrial domains. One of the most significant areas of application is electrosynthesis, which leverages electrochemical processes to synthesize complex organic and inorganic compounds. This method is particularly useful in the production of pharmaceuticals, agrochemicals, and specialty chemicals, offering precise control over reaction conditions and minimizing waste.
Electrolysis processes are another cornerstone of modern electrochemistry, playing a crucial role in the production of essential industrial chemicals such as chlorine and sodium hydroxide in the chlor-alkali industry. Additionally, electrolysis is vital in the extraction of metals like aluminum and in the generation of hydrogen through water electrolysis, which is pivotal for sustainable energy solutions.
Corrosion protection is a critical application where electrochemical principles are employed to safeguard metals from degradation. Techniques such as electroplating, sacrificial anode protection, and anodic protection are widely used to extend the lifespan of metal structures in various environments, from marine settings to industrial facilities.
Electrocatalysis represents a cutting-edge field where electrochemistry intersects with catalysis to enhance the efficiency of chemical reactions. This is particularly important in the development of fuel cells, which convert chemical energy directly into electrical energy with high efficiency and minimal environmental impact.
In the realm of new energy sources, electrochemistry is at the forefront of research into advanced battery technologies, including lithium-ion batteries and solid-state batteries. These technologies are essential for the transition to renewable energy systems, offering higher energy densities and longer lifespans compared to traditional batteries.
Photoelectrochemistry merges photochemistry with electrochemistry, utilizing light energy to drive electrochemical reactions. This field is crucial for the development of solar cells and photoelectrochemical water splitting, which can convert sunlight directly into hydrogen, a clean and abundant fuel.
Lastly, bioelectrochemistry is an emerging discipline that applies electrochemical principles to biological systems. This includes the development of biosensors for medical diagnostics and bioelectrocatalysis for bioremediation and bioenergy production. These applications highlight the profound impact of electrochemistry on both human health and environmental sustainability.
Practical Applications of Electrochemistry
Electrolysis and Electrosynthesis
Electrolysis and electrosynthesis are pivotal in transforming raw materials into valuable products, driving several industrial processes. The chlor-alkali industry stands as a cornerstone, utilizing electrolysis to produce chlorine and sodium hydroxide, essential for various chemical processes and manufacturing sectors. This process not only ensures a steady supply of these chemicals but also underscores the efficiency and scalability of electrochemical methods.
In the realm of aluminum electrolysis, the Hall-Héroult process remains indispensable. This method, which involves the electrolytic reduction of alumina dissolved in molten cryolite, is crucial for the global aluminum production industry. The continuous refinement and optimization of this process have significantly reduced energy consumption and environmental impact, making it a model of sustainable industrial practice.
Water electrolysis has garnered significant attention, particularly in the context of renewable energy storage and hydrogen production. By splitting water into hydrogen and oxygen using an electric current, this process offers a clean and scalable solution for generating hydrogen fuel, which can be stored and used to power various applications, from transportation to industrial processes.
Electrosynthesis extends the scope of electrochemical applications, enabling the synthesis of complex organic molecules and pharmaceuticals. This technique allows for precise control over reaction conditions, leading to higher yields and purity of products. The versatility of electrosynthesis is exemplified by its use in the production of fine chemicals, agrochemicals, and specialty materials, reinforcing its importance in modern chemical manufacturing.
These applications collectively highlight the transformative potential of electrolysis and electrosynthesis, driving innovation across multiple industries and contributing to sustainable development goals.
Metal Corrosion and Protection
Metal corrosion is a significant issue in various industries, leading to substantial economic losses and safety hazards. To mitigate these effects, several advanced methods have been developed and implemented. Among these, electroplating stands out as a versatile technique that not only enhances the aesthetic appeal of metals but also significantly improves their resistance to corrosion. By depositing a thin layer of a more corrosion-resistant metal onto the surface of the base metal, electroplating acts as a protective barrier against environmental elements.
Another effective method is sacrificial anode protection, which involves attaching a more reactive metal to the structure that needs protection. This sacrificial metal, often made of magnesium or zinc, corrodes preferentially, thereby protecting the underlying metal from corrosion. This technique is widely used in marine environments where corrosion rates are particularly high.
Anodic protection is yet another sophisticated approach that involves polarizing a metal to a potential where it becomes passive, significantly reducing its corrosion rate. This method is particularly effective for metals that can form a stable oxide layer, such as stainless steel. By controlling the potential, anodic protection can extend the lifespan of critical infrastructure in industries like chemical processing and oil refining.
These methods, along with others like cathodic protection and coating technologies, collectively form a robust arsenal against metal corrosion, ensuring the longevity and reliability of metallic structures in diverse applications.
Bioelectrochemistry
Bioelectrochemistry represents a fascinating intersection of biology and electrochemistry, with applications that span from medical diagnostics to environmental monitoring. One of the most prominent applications is in the development of biosensors, which leverage biological molecules to detect and quantify specific substances. These sensors are pivotal in medical diagnostics, allowing for the rapid and accurate detection of biomarkers, hormones, and pathogens. For instance, glucose biosensors are widely used in diabetes management, providing real-time monitoring of blood glucose levels.
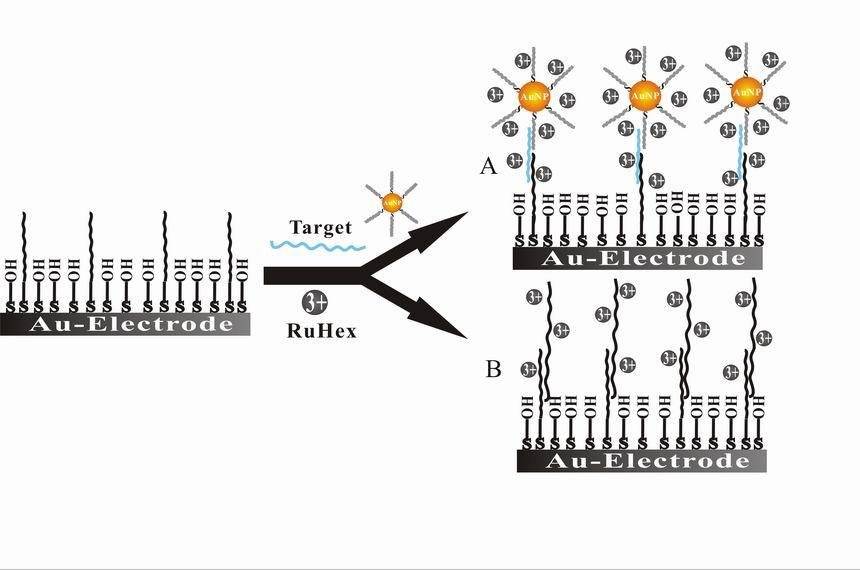
In addition to biosensors, bioelectrocatalysis is another critical area within bioelectrochemistry. This field focuses on using enzymes or microorganisms as catalysts in electrochemical reactions, enhancing the efficiency and specificity of these processes. Bioelectrocatalysis has significant implications in renewable energy, waste treatment, and industrial processes. For example, microbial fuel cells utilize bioelectrocatalysis to convert organic waste directly into electricity, offering a sustainable solution for energy production and waste management.
| Application | Description | Example |
|---|---|---|
| Biosensors | Utilize biological molecules for detection and quantification | Glucose biosensors for diabetes management |
| Bioelectrocatalysis | Use enzymes or microorganisms as catalysts in electrochemical reactions | Microbial fuel cells for energy production from organic waste |
The synergy between biological and electrochemical principles in bioelectrochemistry continues to drive innovation, promising new tools and methods for both scientific research and practical applications.
Chemical Power Supplies
Chemical power supplies encompass a diverse array of technologies, each with unique characteristics and applications. The primary categories include primary batteries, secondary batteries, lithium-ion batteries, and fuel cells. Primary batteries, such as alkaline and zinc-carbon batteries, are designed for single-use and are commonly found in everyday devices like remote controls and flashlights. Secondary batteries, or rechargeable batteries, include lead-acid and nickel-metal hydride types, which can be recharged multiple times, making them ideal for applications requiring sustained power over extended periods.
Lithium-ion batteries have revolutionized portable electronics and electric vehicles due to their high energy density, long lifespan, and low self-discharge rates. These batteries are composed of lithium compounds as electrodes and a non-aqueous electrolyte, offering a balance between cost, performance, and environmental impact.
Fuel cells, on the other hand, represent a significant advancement in sustainable energy technology. Unlike batteries, which store energy chemically, fuel cells generate electricity through the electrochemical reaction of a fuel, typically hydrogen, with an oxidant, usually oxygen. This process results in high efficiency, low pollution, and the ability to adjust power generation based on demand. Fuel cells are particularly promising for applications such as electric vehicles, backup power systems, and even space exploration, where reliable and clean energy is paramount.
| Type of Battery | Primary Use Cases | Key Advantages |
|---|---|---|
| Primary Batteries | Single-use devices (remotes, flashlights) | Cost-effective, widely available |
| Secondary Batteries | Rechargeable devices (phones, laptops) | Reusable, long lifespan |
| Lithium-ion Batteries | Portable electronics, electric vehicles | High energy density, low self-discharge |
| Fuel Cells | Electric vehicles, backup power, space | High efficiency, low pollution, scalable |
Related Products
- Lab Electrochemical Workstation Potentiostat for Laboratory Use
- Gold Disc Electrode
- Gold Electrochemical Sheet Electrode Gold Electrode
- Electrode Fixture for Electrochemical Experiments
- H Type Electrolytic Cell Triple Electrochemical Cell
Related Articles
- Comprehensive Guide to Rotating Disk Electrode (RDE) in Electrochemical Studies
- Understanding Saturated Calomel Reference Electrodes: Composition, Uses, and Considerations
- Exploring the Multifunctional Electrolytic Cell Water Bath: Applications and Benefits
- Revolutionizing Quality Control: The Ultimate Guide to Handheld Lithium Battery Analyzers
- Comprehensive Guide to Reference Electrodes: Types, Applications, and Selection Criteria













