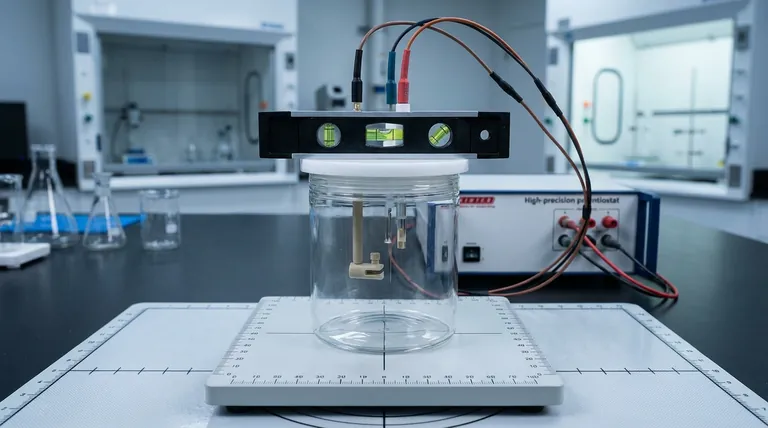
The most dangerous moment in an experiment isn't when the chemicals mix. It isn't when the current spikes.
It is right at the beginning, during the setup.
We often view the physical assembly of the lab apparatus as a chore—a hurdle to clear before the "real" science begins. This is a psychological trap. In electrochemistry, the geometry of your setup is the science.
If your electrolytic cell wobbles, your current density fluctuates. If your electrodes aren't vertical, your results are skewed. The battle for accurate data is won or lost before you ever flip the power switch.
Here is the engineering philosophy behind properly securing your cell, and why stability is the bedrock of discovery.
The Geometry of Certainty
An electrolytic cell is not just a container; it is a controlled universe. To govern this universe, you must first immobilize it.
When you place the cell on the laboratory stand, you are establishing a coordinate system for your experiment.
The Protocol:
- Square Placement: Place the cell firmly on the base. It must be level.
- The Tension Balance: Tighten the fixing knobs. The goal is firm immobility, not crushing force. You want to prevent movement without stressing the glass or housing.
Why does this matter? Because electrochemical reactions occur at the interface of the electrode and the solution. If the cell vibrates or shifts, that interface becomes chaotic. Stability creates the silence required for the signal to emerge.
Verticality and Current Density
In an ideal world, the distance between your working electrode and your counter electrode is perfectly constant.
In the real world, gravity and poor setup conspire against this.
If your cell is tilted, the electrodes hang at an angle. The distance at the top differs from the distance at the bottom. This creates a gradient in current density. Your data will show a "smear" of activity rather than a precise measurement.
Alignment Checklist:
- Vertical Alignment: The cell must be perpendicular to the bench.
- Electrode Spacing: Ensure electrodes hang freely without touching.
- The Dry/Wet Divide: Submerge the active surface fully. Keep the conductive rods at the top bone-dry.
The Chemistry of Control
Once the physical structure is secure, you must stabilize the invisible environment.
Variables are the enemy. If you cannot control the temperature or the purity, you cannot trust the result.
- Purity: Use high-purity reagents. A single rogue ion from tap water can dominate your cyclic voltammetry.
- Volume: Fill to the maximum volume line, but never beyond. Consistency in volume ensures consistency in concentration.
- Atmosphere: If the reaction is sensitive to oxygen, purge with argon or nitrogen. You are creating a vacuum of variables, allowing only the interaction you wish to study.
The Nervous System: Electrical Integrity
The wires connecting your cell to the potentiostat are the nervous system of the experiment.
A crossed wire is not a minor error; it is a fatal stroke to the data.
The "Measure Twice" Rule:
- Check Specs: Does your power supply match the cell’s rating?
- Polarity: Positive to Positive. Negative to Negative. This sounds elementary, yet it remains a leading cause of equipment failure.
- The Power Sequence: Always turn the power supply off before disconnecting cables. Disconnecting a live circuit creates an arc—a microscopic lightning bolt that ruins data and endangers the user.
The Safety Net
Great engineers assume failure is possible and plan for it.
In electrochemistry, this means anticipating the leak. Working with corrosive electrolytes without a secondary containment strategy is a gamble you don't need to take.
Essential Precautions:
- The Pad: Always place a leak-proof pad under the cell. It protects the lab bench and contains hazards.
- The Inspection: Check cables for fraying. A short circuit is a fire hazard.
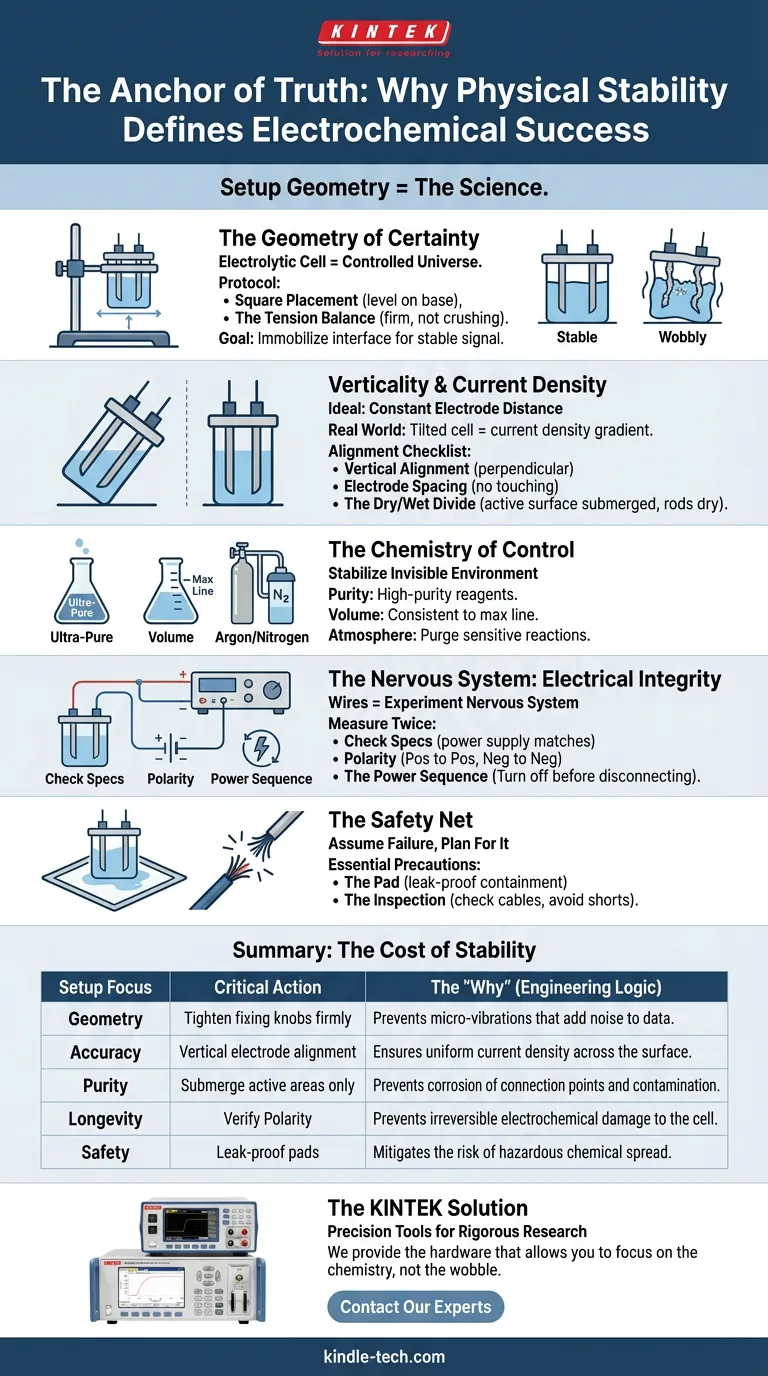
Summary: The Cost of Stability
We often ignore these steps because they feel mundane. But in the lab, boring is good. "Boring" means predictable. "Boring" means safe.
The following table outlines the relationship between setup actions and experimental outcomes:
| Setup Focus | Critical Action | The "Why" (Engineering Logic) |
|---|---|---|
| Geometry | Tighten fixing knobs firmly | Prevents micro-vibrations that add noise to data. |
| Accuracy | Vertical electrode alignment | Ensures uniform current density across the surface. |
| Purity | Submerge active areas only | Prevents corrosion of connection points and contamination. |
| Longevity | Verify Polarity | Prevents irreversible electrochemical damage to the cell. |
| Safety | Leak-proof pads | Mitigates the risk of hazardous chemical spread. |
The KINTEK Solution
Precision is not just about human effort; it is about the quality of the tools you hold in your hands.
A perfectly executed procedure cannot save a poorly manufactured cell. At KINTEK, we understand that our equipment is the vessel for your intellectual property. We design lab equipment and consumables that support the rigor your research demands.
From stable stands to high-precision electrolytic cells, we provide the hardware that allows you to focus on the chemistry, not the wobble.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell with Five-Port
- Super Sealed Electrolytic Electrochemical Cell
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell for Coating Evaluation
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
Related Articles
- The Transparency Paradox: Mastering the Fragile Art of Electrolytic Cells
- The Fragile Vessel of Truth: A Maintenance Manifesto for Electrolytic Cells
- The Silent Variable: Engineering Reliability in Electrolytic Cells
- The Symphony of Coefficients: Why Your Electrolytic Cell Cannot Be a Monolith
- The Architecture of Precision: Mastering Electrolytic Cell Maintenance