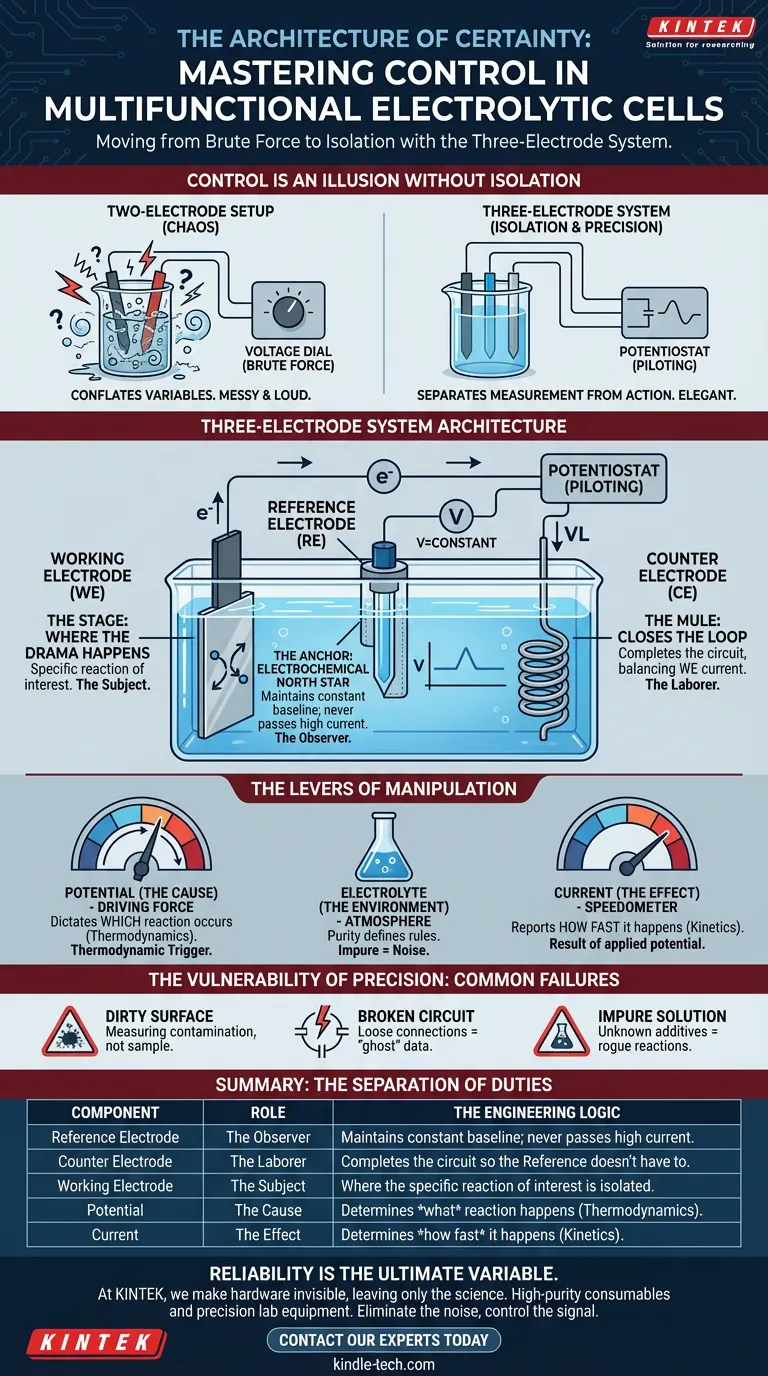
Control is an Illusion Without Isolation
In most complex systems, we confuse effort with control. We assume that if we push harder, the machine will go faster.
In the delicate world of electrochemistry, this mindset is disastrous.
A multifunctional electrolytic cell is a chaotic environment. Electrons are flowing, ions are migrating, and chemical bonds are breaking. If you try to manage this chaos by simply cranking up a voltage dial on a basic power supply, you are not conducting science. You are gambling.
True control requires a shift in philosophy. It requires moving from a system of brute force to a system of isolation.
To achieve this, engineers and chemists utilize a specific hardware configuration: the three-electrode system.
The Engineering Romance of the Three-Electrode System
A standard two-electrode setup conflates the variables. It mixes the driving force of the reaction with the resistance of the solution. It is messy. It is loud.
The three-electrode system is elegant because it separates measurement from action. It allows you to define the "why" (potential) independently of the "how fast" (current).
Here is how the architecture of precision is built:
1. The Stage: The Working Electrode (WE)
This is where the drama happens. Whether you are studying corrosion, depositing a film, or synthesizing a molecule, it happens on this surface. Everything in the experiment is designed to serve this single interface.
2. The Anchor: The Reference Electrode (RE)
This is the cornerstone of accuracy.
The Reference Electrode acts as a stable electrochemical "North Star." It has a known, constant potential. It does not participate in the heavy lifting of current flow. Its only job is to sit quietly and provide a baseline against which the Working Electrode is measured.
3. The Mule: The Counter Electrode (CE)
The Counter Electrode exists to close the loop. Current must flow somewhere.
The CE accepts the current balancing the reaction at the WE. By dumping the electrical load onto the Counter Electrode, the system protects the delicate Reference Electrode from destabilizing currents.
The Levers of Manipulation
Once you have established this hardware triad, you are no longer guessing. You are piloting.
You now have direct access to the thermodynamic levers of the universe.
The Driving Force (Potential)
By controlling the voltage difference between the Working and Reference electrodes, you dictate exactly which reaction occurs.
This is the "thermodynamic trigger." Set the potential too low, and nothing happens. Set it just right, and you activate only the specific chemical pathway you desire, ignoring all others.
The Speed (Current)
Current flows between the Working and Counter electrodes.
This is your speedometer. It tells you the rate of the reaction. In a properly configured cell, the current is a result of your applied potential. You set the cause; the current reports the effect.
The Environment (Electrolyte)
The electrolyte is the atmosphere of your reaction. Its purity and concentration define the rules of the game. An impure electrolyte is like trying to listen to a whisper in a thunderstorm—the signal is lost in the noise.
The Vulnerability of Precision
There is a psychological trap in using high-precision equipment. We tend to trust the digital display more than the physical reality.
A potentiostat is a brilliant machine, but it cannot correct for physical negligence. The theoretical perfection of the three-electrode system collapses under three common failures:
- The Dirty Surface: If your Working Electrode is contaminated, you are measuring the contamination, not your sample.
- The Broken Circuit: A loose connection or a cracked cell creates resistance that mimics chemical behavior. It generates "ghost" data.
- The Impure Solution: Unknown additives in your electrolyte act as rogue actors, catalyzing reactions you didn't ask for.
Precision is not just about having the right machine. It is about the discipline of preparation.
Summary: The Separation of Duties
To understand the system at a glance, view the separation of duties in this breakdown:
| Component | Role | The Engineering Logic |
|---|---|---|
| Reference Electrode | The Observer | Maintains a constant baseline; never passes high current. |
| Counter Electrode | The Laborer | Completes the circuit so the Reference doesn't have to. |
| Working Electrode | The Subject | Where the specific reaction of interest is isolated. |
| Potential | The Cause | Determines what reaction happens (Thermodynamics). |
| Current | The Effect | Determines how fast it happens (Kinetics). |
Reliability is the Ultimate Variable
In the laboratory, the most expensive commodity is not the equipment—it is the researcher's time.
Hours spent troubleshooting a drifting reference electrode or analyzing data from a contaminated cell are hours lost forever. The goal of the three-electrode system is to make the hardware invisible, leaving only the science.
At KINTEK, we understand that engineers and scientists need tools that disappear into the background. We specialize in the high-purity consumables and precision lab equipment that allow the three-electrode system to function as intended.
From leak-proof electrolytic cells to surface-perfect electrodes, our equipment is built to eliminate the noise so you can control the signal.
Contact our experts today to secure the foundation of your next experiment.
Visual Guide
Related Products
- Lab Electrochemical Workstation Potentiostat for Laboratory Use
- Super Sealed Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell for Coating Evaluation
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
Related Articles
- Advanced Techniques in Coating Evaluation Using Electrolytic Cells
- Exploring the Multifunctional Electrolytic Cell Water Bath: Applications and Benefits
- Ultimate Guide to Handheld Alloy Analyzers: Features, Applications, and Advantages
- Revolutionizing Quality Control: The Ultimate Guide to Handheld Lithium Battery Analyzers
- Comprehensive Guide to Reference Electrodes: Types, Applications, and Selection Criteria




















