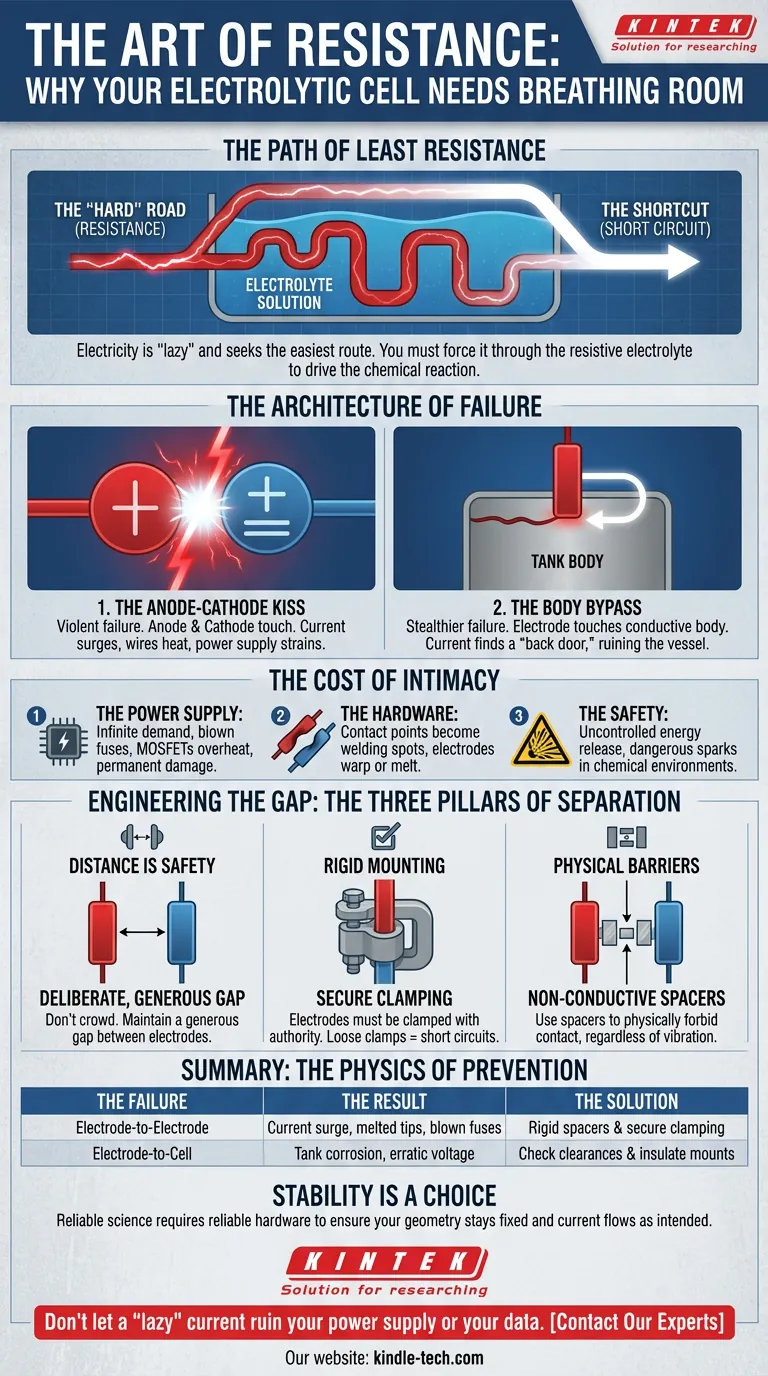
The Path of Least Resistance
Electricity is fundamentally lazy. It is a force of nature that constantly seeks the easiest route from point A to point B.
In an electrolytic cell, your job is to act as a dam builder. You are forcing electricity to take the "hard" road—traveling through the resistive electrolyte solution to drive a chemical reaction.
When you fail to maintain that resistance, the current finds a shortcut. This is the short circuit. It is not just a technical glitch; it is the electricity bypassing your instructions.
To prevent this, we must understand the physical geometry of the cell. The safety of your experiment relies on the empty space between components as much as the components themselves.
The Architecture of Failure
A short circuit is rarely a mystery. It is almost always a failure of spatial discipline.
When the intended high-resistance path (the electrolyte) is bypassed by a zero-resistance path (direct contact), the system collapses. The power supply, seeing no resistance, dumps its maximum current instantly.
Here is how the architecture fails:
1. The Anode-Cathode Kiss
This is the most violent failure. The anode and cathode are meant to be partners, not lovers.
If they touch, even for a millisecond, the electrolyte is removed from the equation. The current surges. The wires heat up. The power supply strains against its own limitations.
2. The Body Bypass
This is the stealthier failure.
If an electrode touches the conductive body of the tank or cell, the current finds a "back door." It might skip the reaction entirely or enter the cell wall, corroding the equipment and ruining the vessel.
The Cost of Intimacy
Why does this matter? Because in high-amperage systems, the consequences of contact are thermodynamic, not just electrical.
- The Power Supply: It tries to feed an infinite demand. Fuses blow. MOSFETs overheat. The unit can be permanently destroyed.
- The Hardware: The point of contact becomes a welding spot. Electrodes warp or melt.
- The Safety: A short circuit is an uncontrolled release of energy. In a lab environment containing chemicals, sparks are unacceptable.
Engineering the Gap
Prevention is not about hope; it is about rigidity.
You cannot rely on manual positioning alone. Gravity, thermal expansion, and the bubbling vibration of electrolysis all conspire to move your electrodes together.
You must engineer the gap.
The Three Pillars of Separation
- Distance is Safety: Don't crowd the cell. Maintain a deliberate, generous gap between the anode and cathode.
- Rigid Mounting: Electrodes should not dangle. They must be clamped with authority. A loose clamp is a short circuit waiting to happen.
- Physical Barriers: Use non-conductive spacers. These are small plastic or ceramic guides that physically forbid the electrodes from touching, regardless of vibration.
Summary: The Physics of Prevention
| The Failure | The Result | The Solution |
|---|---|---|
| Electrode-to-Electrode | Current surge, melted tips, blown fuses | Rigid spacers & secure clamping |
| Electrode-to-Cell | Tank corrosion, erratic voltage | Check clearances & insulate mounts |
Stability is a Choice
The difference between a failed experiment and a breakthrough often comes down to the quality of the setup.
At KINTEK, we understand that reliable science is built on reliable hardware. We provide the lab equipment and consumables that ensure your geometry stays fixed, your spacing stays true, and your current flows exactly where you intend it to.
Don't let a "lazy" current ruin your power supply or your data.
Visual Guide
Related Products
- Optical Water Bath Electrolytic Electrochemical Cell
- Super Sealed Electrolytic Electrochemical Cell
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
Related Articles
- The Glass Heart of the Experiment: Precision Through Systematic Care
- The Transparency Paradox: Mastering the Fragile Art of Electrolytic Cells
- The Quiet Discipline: Mastering the Post-Use Protocol for Five-Port Electrolytic Cells
- The Architecture of Precision: Mastering Electrolytic Cell Maintenance
- Understanding Electrolytic Cells and Their Role in Copper Purification and Electroplating