The Silent Variable in the Lab
In scientific research, we obsess over the variables we can see. We scrutinize the purity of the catalyst. We calibrate the voltage to the microvolt. We obsess over the reaction mechanism.
But we often ignore the environment where these interactions happen.
The electrolytic cell is often treated as a mere bucket—a passive container for fluids. This is a mistake. In electrochemistry, the geometry of the vessel is not just a container; it is a component of the circuit.
The standard multifunctional electrolytic cell is designed to solve a specific problem: how to create a controlled, reproducible environment for the three-electrode system. It is the invisible baseline upon which reliable data is built.
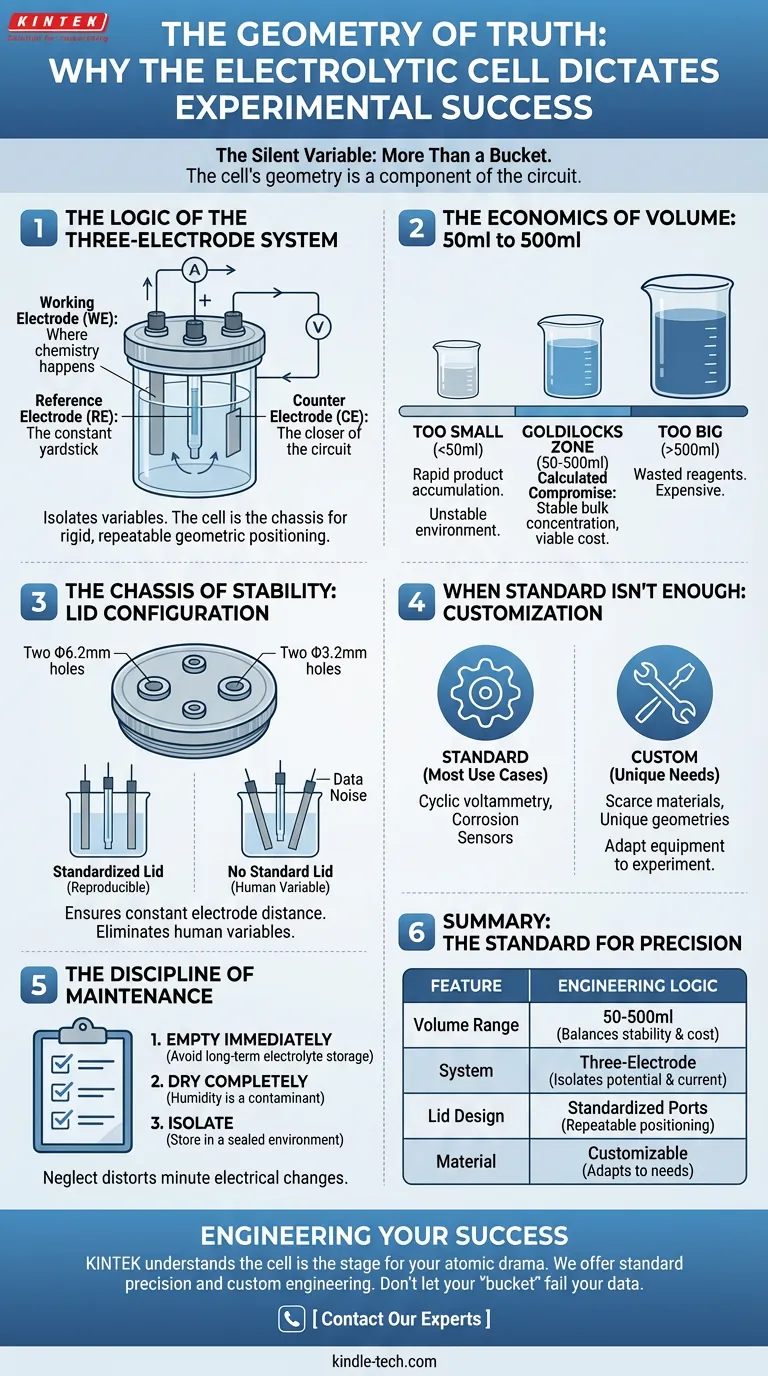
The Logic of the Three-Electrode System
To understand the cell, you must understand what it protects.
Modern electrochemistry relies on the three-electrode setup. It is a brilliant system designed to isolate variables.
- The Working Electrode: Where the chemistry happens.
- The Reference Electrode: The constant yardstick against which potential is measured.
- The Counter Electrode: The closer of the circuit, allowing current to flow without disturbing the reference.
If these three components are not held in a rigid, repeatable geometric relationship, your data becomes noise. The multifunctional cell is the chassis that holds this system in place.
The Economics of Volume: 50ml to 500ml
Why is the standard volume range between 50ml and 500ml?
It isn't an arbitrary number. It is a calculated compromise between chemical stability and economic reality.
The Risk of "Too Small": If a cell is too small, the reaction products accumulate rapidly. The concentration of the electrolyte shifts within seconds. The environment changes faster than you can measure it, rendering steady-state experiments impossible.
The Risk of "Too Big": If the cell is massive, you waste reagents. In modern research, electrolytes and catalysts are often exotic and expensive. A 500ml limit allows for a "Goldilocks" zone—large enough to maintain a stable bulk concentration, but small enough to be viable for routine lab work.
The Chassis of Stability: Lid Configuration
The most underrated component of the cell is the lid.
In a standard configuration, you will find specific apertures:
- Two Φ6.2mm holes
- Two Φ3.2mm holes
These are not random drill holes. They are standardized docking ports. They ensure that the distance between the working electrode and the counter electrode remains constant from Experiment A to Experiment B.
Without a standardized lid, electrode placement becomes a human variable. And human variables are the enemy of reproducibility.
When Standard Isn't Enough
While the 50-500ml range covers 90% of use cases—cyclic voltammetry, corrosion testing, sensor development—science is rarely one-size-fits-all.
Sometimes you are working with a material so scarce that 50ml is too much. Sometimes your electrode geometry is unique.
This is where the distinction between a commodity and a tool becomes clear. High-quality lab equipment providers allow for customization. You can alter the cell volume or request custom aperture sizes to fit non-standard probes. The equipment should adapt to the experiment, not the other way around.
The Discipline of Maintenance
The greatest threat to an electrolytic cell is not breakage; it is neglect.
Because these cells measure minute electrical changes, even a trace of dried salt or a microscopic layer of corrosion can act as a resistor or a capacitor, distorting your data.
The protocol is simple but strict:
- Empty immediately: Never leave electrolytes in the cell for long-term storage.
- Dry completely: Humidity is a contaminant.
- Isolate: Store in a sealed environment.
Summary: The Standard for Precision
| Feature | Typical Specification | Engineering Logic |
|---|---|---|
| Volume Range | 50ml to 500ml | Balances chemical stability with reagent costs. |
| System | Three-Electrode | Isolates potential control from current flow. |
| Lid Design | Φ6.2mm & Φ3.2mm ports | Ensures repeatable geometric positioning. |
| Material | Customizable | Adapts to unique chemical compatibility needs. |
Engineering Your Success
At KINTEK, we understand that an electrolytic cell is more than glass and Teflon. It is the stage where your atomic drama unfolds.
We provide standard 50ml-500ml cells for routine precision, but we also possess the engineering capability to customize vessels for your specific constraints. Whether you need to conserve expensive materials or accommodate unique electrode geometries, we build the vessel that fits your science.
Don't let your "bucket" be the reason your data fails.
Visual Guide
Related Products
- Super Sealed Electrolytic Electrochemical Cell
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- H Type Electrolytic Cell Triple Electrochemical Cell
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
Related Articles
- The Architecture of Control: Mastering the Super-Sealed Electrolytic Cell
- The Architecture of Precision: Mastering the Five-Port Water Bath Electrolytic Cell
- The Anchor of Truth: Why Physical Stability Defines Electrochemical Success
- The Architecture of Precision: Why the Invisible Details Define Electrochemical Success
- The Transparency Paradox: Mastering the Fragile Art of Electrolytic Cells




















