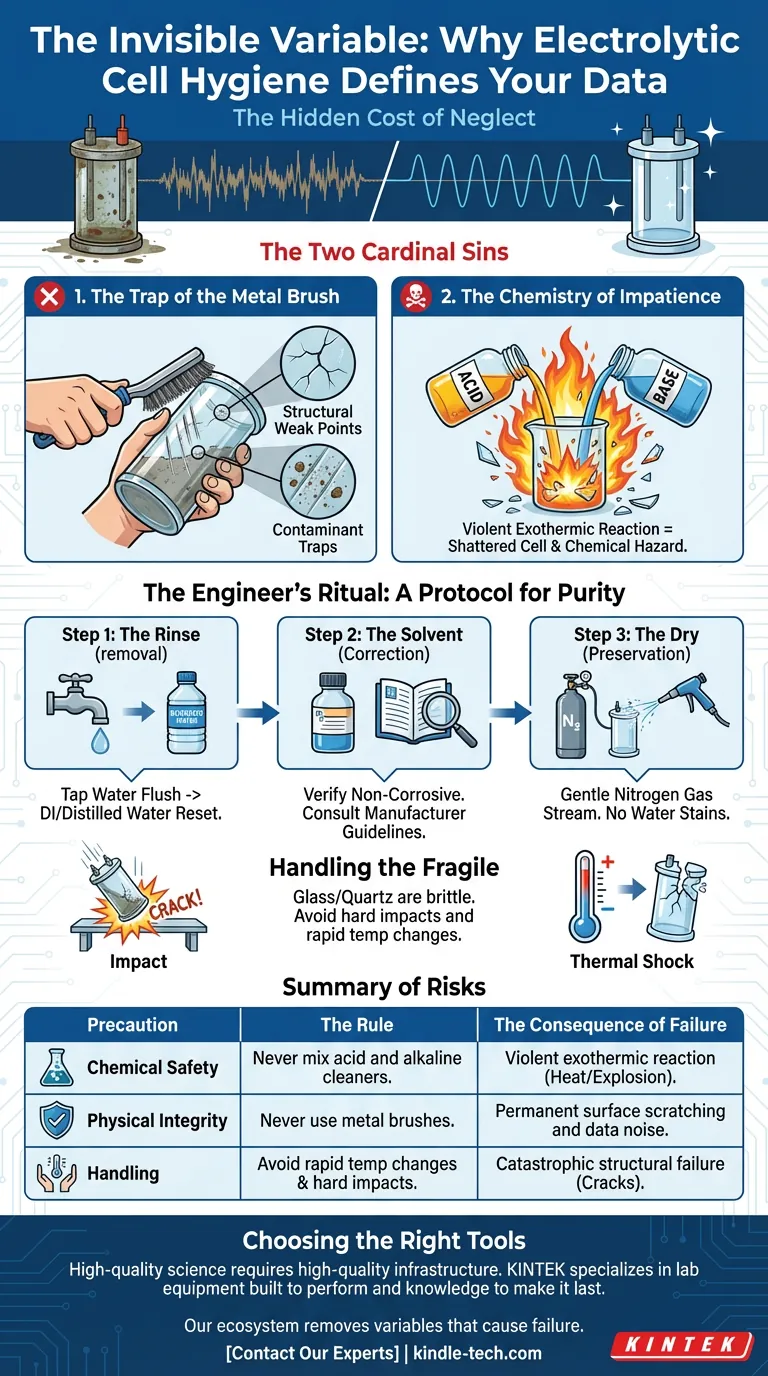
The Hidden Cost of Neglect
In the laboratory, we often obsess over the purity of our reagents and the precision of our power supplies. We treat the experiment itself as the main event.
But the cleanup? That is often treated as a chore. An afterthought.
This is a dangerous psychological blind spot.
An electrolytic cell is not merely a container; it is an active participant in your electrochemical process. Its physical integrity and surface cleanliness are variables that directly feed into your data. If you ignore the maintenance, you aren't just making a mess. You are introducing noise into your results.
More importantly, you are introducing risk to your life.
The Two Cardinal Sins
When maintaining these delicate instruments, most failures stem from two specific behaviors: impatience and aggression.
1. The Trap of the Metal Brush
It is tempting to scrub. When we see residue, our instinct is to use force.
However, using a metal brush on an electrolytic cell is fatal to the equipment.
Whether the cell is glass, quartz, or contains coated electrodes, the surface is microscopically precise. A metal brush does not just clean; it gouges. It leaves permanent scratches.
These scratches are not just cosmetic issues. They become:
- Structural weak points that lead to cracks under pressure.
- Contaminant traps where ions hide, ruining future experiments.
2. The Chemistry of Impatience
The second sin is mixing cleaning agents without thought.
There is a tendency to believe that if an acid cleans well, and a base cleans well, using them in sequence (or worse, together) will clean perfectly.
This is false. Mixing acids (like nitric acid) and bases (like sodium hydroxide) triggers a violent exothermic reaction.
The resulting heat can shatter the cell instantly. It creates a chemical hazard that endangers the operator. Chemical safety is not about complex formulas; it is about respecting the simple rule of separation.
The Engineer’s Ritual: A Protocol for Purity
To maintain the "romance" of precise engineering—where tools work exactly as intended—we must treat cleaning as a ritual, not a chore.
Adopt this structured workflow to protect the cell's integrity.
Step 1: The Rinse (removal)
Start simple. Use standard tap water to flush out the bulk of electrolytes and reagents.
Follow immediately with deionized or distilled water. This is the reset button. It strips away the ions introduced by the tap water.
Step 2: The Solvent (Correction)
If residue persists, use a cleaning agent. But pause here.
You must verify that the chemical is non-corrosive to your specific cell material. An engineer knows the limits of their materials. Consult the manufacturer’s guidelines. If you guess, you lose.
Step 3: The Dry (Preservation)
Water stains are minerals left behind. Minerals are contaminants.
Do not let the cell air dry if precision matters. Use a gentle stream of nitrogen gas. This ensures the surface is physically dry and chemically neutral, ready for the next measurement.
Handling the Fragile
The body of an electrolytic cell is typically fashioned from glass or quartz.
These materials offer optical transparency and chemical resistance, but they pay for it with brittleness.
- Impact: A minor collision with a lab bench can render a thousand-dollar piece of equipment useless.
- Thermal Shock: Moving a quartz cell from a hot bath to a cold rinse will cause it to fracture. Glass cannot handle the stress of rapid expansion and contraction.
Allow the equipment to breathe. Let it return to room temperature gradually.
Summary of Risks
The following table outlines the relationship between behavior and consequence.
| Precaution | The Rule | The Consequence of Failure |
|---|---|---|
| Chemical Safety | Never mix acid and alkaline cleaners. | Violent exothermic reaction (Heat/Explosion). |
| Physical Integrity | Never use metal brushes. | Permanent surface scratching and data noise. |
| Handling | Avoid rapid temp changes & hard impacts. | Catastrophic structural failure (Cracks). |
Choosing the Right Tools
Your data is only as good as the vessel it is generated in.
If your focus is safety, your protocol must ban the mixing of incompatible chemicals. If your focus is accuracy, your protocol must obsess over nitrogen drying and deionized rinsing.
At KINTEK, we understand that high-quality science requires high-quality infrastructure. We specialize in lab equipment that is built to perform, but we also provide the knowledge to ensure that equipment lasts.
From durable electrolytic cells to the specific consumables needed to maintain them, our ecosystem is designed to remove the variables that cause failure.
Do not let a dirty cell be the reason your hypothesis fails.
Visual Guide
Related Products
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
- Customizable PEM Electrolysis Cells for Diverse Research Applications
- Side Window Optical Electrolytic Electrochemical Cell
- Thin-Layer Spectral Electrolysis Electrochemical Cell
- Battery Lab Equipment Battery Capacity and Comprehensive Tester
Related Articles
- The Vessel of Truth: Why the Container Matters More Than the Chemistry
- PTFE's high temperature and corrosion resistance: Why it is indispensable in industry
- The Silent Variable: Engineering Reliability in Electrolytic Cells
- The Unseen Variable: Mastering the Electrolytic Cell Inspection
- The Art of Resistance: Why Your Electrolytic Cell Needs Breathing Room




















