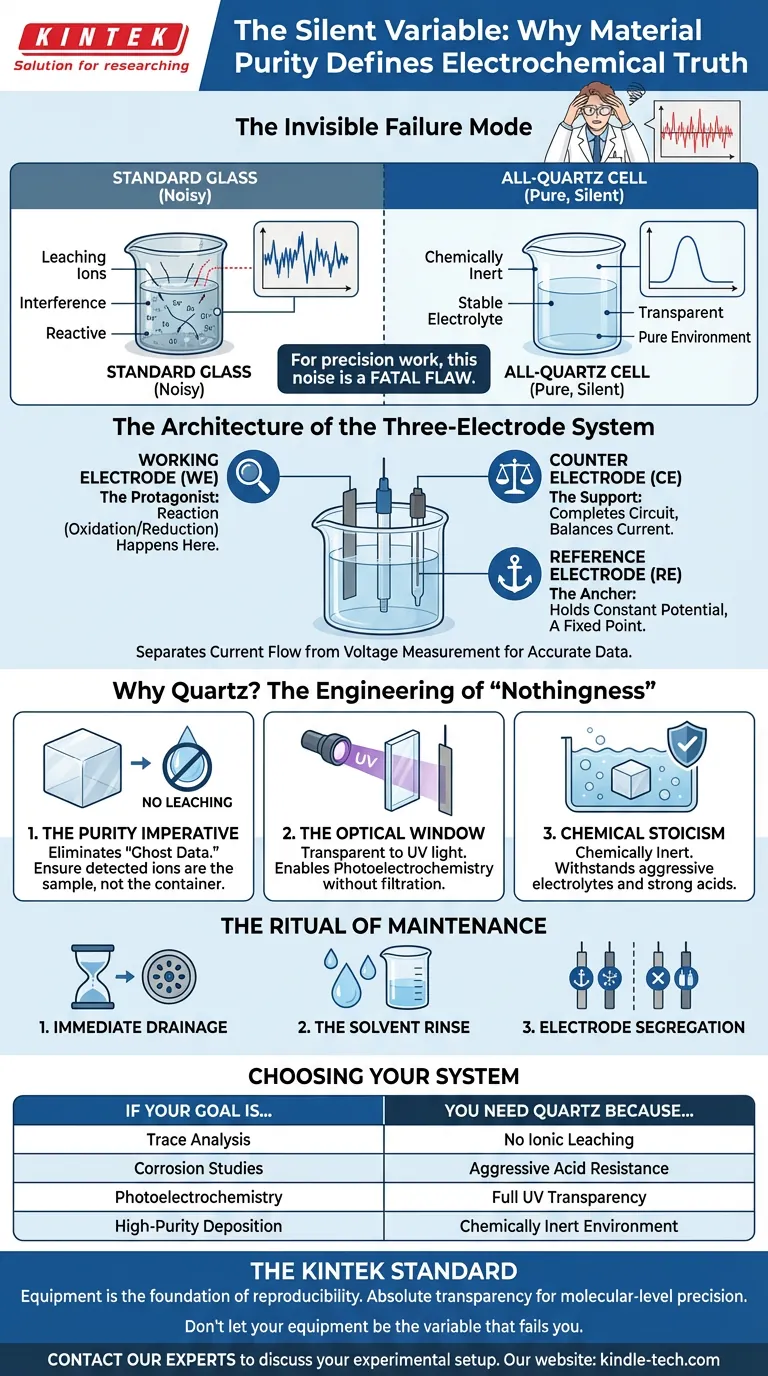
The Invisible Failure Mode
In experimental science, we obsess over variables. We calibrate the potentiostat. We polish the electrodes. We purify the electrolytes.
We treat the experiment like a controlled performance, where every actor has a script.
But often, we ignore the stage itself.
In electrochemistry, the vessel holding your solution is not a passive container. It is an environment. If you are using standard borosilicate glass for sensitive work, that environment is chemically "noisy." It leaches ions. It reacts. It interferes.
For routine work, this noise is background static. For precision work—like trace analysis or photoelectrochemistry—it is a fatal flaw.
This is why the all-quartz electrolytic cell exists. It is designed for a specific type of anxiety: the fear that the container is altering the result.
The Architecture of the Three-Electrode System
Before understanding the material, we must understand the mechanics.
The all-quartz cell is the housing for the three-electrode system, the standard configuration for studying reaction kinetics. It separates the flow of current from the measurement of voltage, preventing the "drift" that ruins data.
Think of it as a triangular relationship:
- The Working Electrode (WE): The protagonist. This is where the reaction happens—oxidation, reduction, or deposition.
- The Counter Electrode (CE): The support. It completes the circuit, balancing the current generated at the working electrode so the system remains neutral.
- The Reference Electrode (RE): The anchor. It holds a constant potential, giving you a fixed point against which to measure the working electrode.
Without this separation, you are measuring the resistance of the wires, not the reaction of the chemistry.
Why Quartz? The Engineering of "Nothingness"
The goal of a high-end electrolytic cell is to be invisible. You want to measure the sample, not the glass.
Standard glass is a mixture. It contains silica, but also boron, sodium, and other additives to make it easier to melt and shape. In aggressive chemical environments, these additives don't stay put. They leach out.
Quartz (fused silica) is different. It is the closest we get to material silence.
1. The Purity Imperative
In trace analysis, a few parts per billion of leached sodium or boron can look exactly like the signal you are hunting for. Quartz eliminates this "ghost data." It ensures that the ions you detect are the ions you put there.
2. The Optical Window
There is a romance to photoelectrochemistry—using light to drive chemical change.
Standard glass blocks ultraviolet (UV) light. It is opaque to the very energy source many experiments require. Quartz is transparent across a broad spectrum, including UV. It allows the cell to act as a window, letting light interact directly with the electrode surface without filtration.
3. Chemical Stoicism
Corrosive media destroy standard equipment. Quartz is chemically inert. It withstands strong acids and aggressive electrolytes that would etch or cloud borosilicate glass. It survives where other materials degrade.
The Ritual of Maintenance
A precision tool requires precision care. An all-quartz cell is robust chemically but requires procedural discipline to maintain its baseline purity.
Treat the cleanup as part of the data collection process:
- Immediate Drainage: Never leave the electrolyte sitting after the current is cut. Drain it immediately to prevent deposition or slow-reaction byproducts from sticking to the walls.
- The Solvent Rinse: Wash with deionized water, followed by a high-purity solvent. The goal is to return the surface to a neutral state.
- Electrode Segregation: Never store the electrodes inside the cell. They have different storage needs (some wet, some dry), and leaving them together risks cross-contamination.
Choosing Your System
Not every experiment needs quartz. But the ones that do, really do.
If you are teaching basic undergraduate chemistry, standard glass is fine. But if you are pushing the boundaries of material science, the choice becomes binary.
Here is the decision matrix:
| If your goal is... | You need Quartz because... |
|---|---|
| Trace Analysis | You cannot afford ionic leaching from the glass. |
| Corrosion Studies | You are using aggressive acids that eat standard glass. |
| Photoelectrochemistry | You need full UV transparency to irradiate the sample. |
| High-Purity Deposition | You need a chemically inert environment to prevent defects. |
The KINTEK Standard
At KINTEK, we understand that equipment is not just hardware; it is the foundation of reproducibility.
When you are working at the molecular level, there is no room for "good enough." You need a vessel that offers absolute transparency—both optically and chemically.
Our all-quartz electrolytic cells are engineered for researchers who cannot afford to question their equipment. Whether you are conducting fundamental electrochemical studies or advanced spectroelectrochemistry, we provide the clean slate your data deserves.
Don't let your equipment be the variable that fails you.
Contact Our Experts to discuss your experimental setup and secure the precision your research demands.
Visual Guide
Related Products
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Electrolytic Electrochemical Cell with Five-Port
- Super Sealed Electrolytic Electrochemical Cell
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell for Coating Evaluation
Related Articles
- The Silent Dialogue: Mastering Control in Electrolytic Cells
- The Invisible Variable: Why Post-Experiment Rituals Define Scientific Truth
- The Glass Heart of the Experiment: Precision Through Systematic Care
- The Glass Heart: Why Good Science Dies in Dirty Cells
- Understanding Quartz Electrolytic Cells: Applications, Mechanisms, and Advantages




















