The Illusion of Simplicity
In laboratory science, we often mistake a procedure for a checklist. We believe that if we follow steps A, B, and C, result D is guaranteed.
But in photoelectrochemistry, reality is messier.
Using a side-window optical electrolytic cell isn't just about mixing chemicals. It is a high-stakes balancing act involving three distinct physical systems:
- The Chemical Environment: The electrolyte and the reaction.
- The Optical Path: The journey of the photon from source to surface.
- The Electronic Measurement: The data captured by the potentiostat.
A failure in any single one of these systems doesn't just reduce efficiency. It renders the entire experiment meaningless.
Here is how to master the invisible variables that define your success.
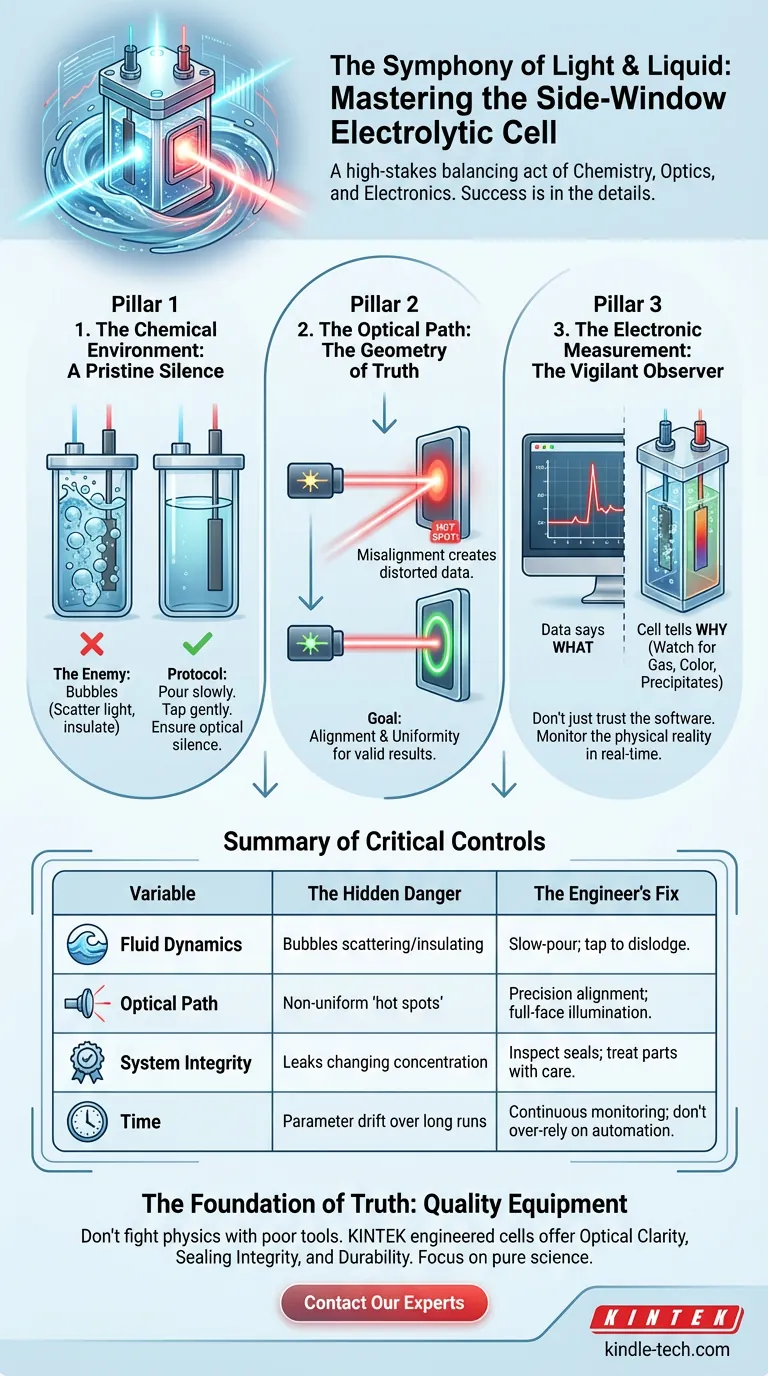
The First Pillar: A Pristine Silence
Before you turn on a laser or connect a wire, you must conquer the physical environment inside the cell.
The enemy here is air.
When you introduce the electrolyte, you are disrupting a static system. If you pour rapidly, you introduce turbulence. Turbulence creates bubbles.
In standard chemistry, a bubble is an annoyance. In photoelectrochemistry, a bubble is a lie.
If a bubble adheres to the optical window, it becomes a lens, scattering your light source before it ever hits the target. If it adheres to the electrode, it becomes an insulator, creating a "dead zone" where no reaction occurs.
The Protocol:
- Pour slowly. Let the liquid rise gently.
- Inspect the window and electrode surfaces from multiple angles.
- If you see a bubble, tap the cell gently. Do not proceed until the system is optically silent.
The Second Pillar: The Geometry of Truth
The defining feature of this cell is the side window. It is the portal through which energy enters your system.
But light is tricky. It doesn't inherently want to be uniform.
Your task is alignment. You must guide the light source—be it a laser or a solar simulator—through the precise center of that window.
But hitting the window is only half the battle. You must ensure the light spot uniformly illuminates the working electrode.
If your alignment is off, you create "hot spots." Parts of your sample will react violently while others remain dormant. The current you measure will be an average of these extremes, giving you data that is mathematically correct but scientifically worthless.
The Third Pillar: The Vigilant Observer
Once the chemistry is pure and the optics are aligned, you connect the leads.
This is the moment most researchers relax. They hit "Start" on the software and walk away to get coffee.
This is a mistake.
The most critical data often isn't on the screen; it is in the cell itself. You must monitor the physical state of the reaction in real-time.
What to watch for:
- Gas Generation: Is it excessive? Is it happening where it shouldn't?
- Color Shifts: Is the electrolyte degrading?
- Precipitates: Are solids forming that will block the light path?
If the software shows a spike, look at the cell. The data tells you what happened; the cell tells you why.
Summary of Critical Controls
The difference between a failed experiment and a breakthrough often lies in the details we ignore.
| Variable | The Hidden Danger | The Engineer's Fix |
|---|---|---|
| Fluid Dynamics | Bubbles scattering light or insulating the electrode. | Slow-pour filling; tap to dislodge voids. |
| Optical Path | Non-uniform beams creating reaction "hot spots." | Precision alignment; check for full-face illumination. |
| System Integrity | Leaks changing concentration or damaging gear. | Inspect seals; treat quartz/glass with extreme care. |
| Time | Parameter drift (temp/concentration) over long runs. | Continuous monitoring; do not rely solely on automation. |
The Role of Equipment in Scientific Truth
There is a romance to engineering a perfect experiment. It is the feeling of eliminating noise until only the signal remains.
But you cannot fight physics with poor tools.
If your cell leaks, no amount of careful pouring will save the data. If your optical window is low-quality, no amount of alignment will fix the scattering.
KINTEK understands that your equipment is the foundation of your data. We specialize in lab equipment and consumables designed for the rigors of serious research.
Our optical electrolytic cells are engineered for:
- Optical Clarity: High-quality windows that respect the path of your photons.
- Sealing Integrity: Robust designs to prevent the drift and danger of leaks.
- Durability: Materials built to withstand the realities of the lab bench.
When you remove the variables caused by the hardware, you are left with the pure science.
Contact Our Experts today to discuss your experimental setup. Let us help you build a system where the only surprises are the discoveries you make.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell with Five-Port
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- Super Sealed Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell for Coating Evaluation
- Side Window Optical Electrolytic Electrochemical Cell
Related Articles
- The Invisible Architecture of Accuracy: Optimizing the Five-Port Electrolytic Cell
- The Silent Dialogue: Mastering Control in Electrolytic Cells
- The Architecture of Precision: Why the Invisible Details Define Electrochemical Success
- The Symphony of Coefficients: Why Your Electrolytic Cell Cannot Be a Monolith
- The Architecture of Precision: Mastering Electrolytic Cell Maintenance