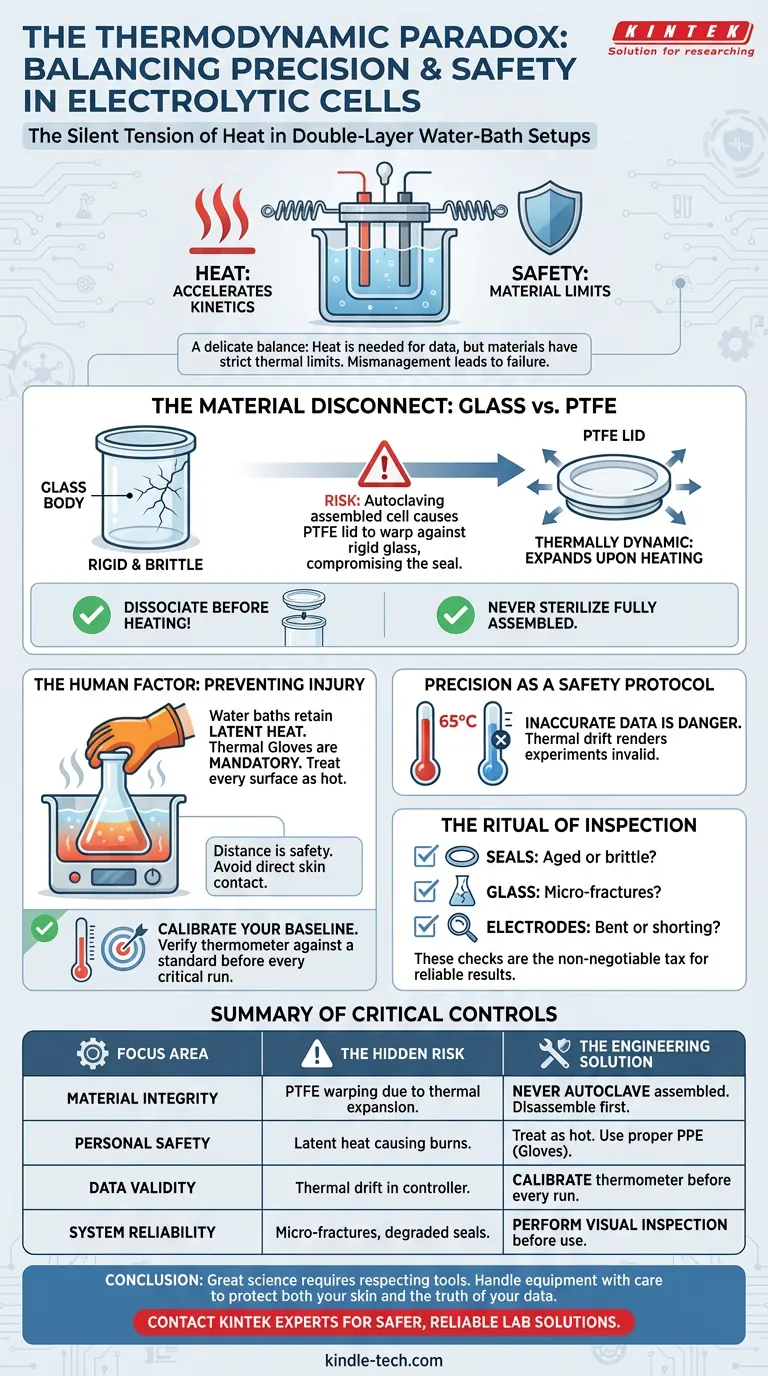
The Silent Tension of Heat
There is a quiet paradox in the laboratory. We use heat to drive reactions, to accelerate kinetics, and to simulate environments. Yet, that same energy, if mismanaged, becomes the enemy of both the apparatus and the operator.
In the context of a double-layer water-bath electrolytic cell, this tension is palpable.
On one hand, you need heat. You need a stable, elevated temperature to validate your electrochemical data. On the other hand, the materials you are using have strict thermal limits.
Safety in this environment is not just about avoiding burns. It is about understanding the "personality" of your materials. It is an engineering romance between the rigidity of glass and the fluidity of thermal expansion.
The Material Disconnect: Glass vs. PTFE
The most common failure in electrolytic setups usually stems from a single, flawed assumption: That the cell is a single unit.
It is not. It is an assembly of disparate materials.
The body is often glass. The lid is frequently PTFE (Teflon).
Glass is rigid and brittle. PTFE is chemically resistant but thermally dynamic. When you heat PTFE, it expands. When it cools, it contracts.
If you autoclave or aggressively heat a fully assembled cell, the PTFE lid will expand against the glass threads or clamps. Upon cooling, it often refuses to return to its original shape.
The result? A warped lid. A compromised seal. A ruined cell.
To protect the integrity of your equipment:
- Dissociate before heating: Never sterilize the cell fully assembled.
- Respect the thermal limit: Understand that the lid and the body react to heat at different rates.
The Human Factor: Preventing Physical Injury
Complexity is the enemy of safety. When an experiment becomes complex, we tend to focus on the data and forget the physical reality of the machine.
The water bath apparatus is a thermal mass. It holds heat long after the controller is turned off.
The precautions here are simple, yet often ignored during the rush of discovery:
- Thermal Gloves are mandatory. Not just for handling the cell, but for adjusting the bath.
- Distance is safety. Avoid direct skin contact with any component connected to the thermal loop.
We often assume we are too smart to get burned. But fatigue and distraction make us all vulnerable.
Precision as a Safety Protocol
Inaccurate data is a form of danger. It leads to false conclusions and wasted resources.
A water bath that reads $60^{\circ}\text{C}$ but delivers $65^{\circ}\text{C}$ is not just an annoyance; it is a variable that renders your experiment invalid.
Calibration is your baseline. Before the experiment begins, verify the water bath’s thermometer against an external standard.
If the environment is not stable, the data is noise. And in science, noise is the ultimate failure.
The Ritual of Inspection
Atul Gawande famously argued that the simple checklist is the most powerful tool in medicine. The same applies to electrochemistry.
A cell does not fail suddenly; it fails gradually.
- The Seals: Are they aged? Are they brittle?
- The Glass: Are there micro-fractures invisible to a glancing eye?
- The Electrodes: Are they bent? Will they short-circuit against the wall?
These checks are not "extra work." They are the work. They are the non-negotiable tax we pay for reliable results.
Summary of Critical Controls
Here is how to align your safety protocols with your experimental goals:
| Focus Area | The Hidden Risk | The Engineering Solution |
|---|---|---|
| Material Integrity | PTFE warping due to thermal expansion. | Never autoclave the fully assembled cell. Disassemble first. |
| Personal Safety | Latent heat in the water bath causing burns. | Treat every surface as hot until proven otherwise. Use proper PPE. |
| Data Validity | Thermal drift in the water bath controller. | Calibrate the thermometer before every critical run. |
| System Reliability | Micro-fractures and degraded seals. | Perform a visual inspection of glass and O-rings before use. |
Conclusion
Great science requires a respect for the tools we use. It requires acknowledging that a glass cell and a PTFE lid are not just holders for liquid, but engineered components with distinct physical limits.
By respecting the temperature, calibrating your tools, and handling your equipment with the care of a craftsman, you protect more than just your skin. You protect the truth of your data.
At KINTEK, we understand the nuances of laboratory materials. We build equipment designed to withstand the rigors of precise thermal regulation. Whether you need robust electrolytic cells or high-precision water baths, our experts can help you build a safer, more reliable lab environment.
Visual Guide
Related Products
- Multifunctional Electrolytic Electrochemical Cell Water Bath Single Layer Double Layer
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Customizable PEM Electrolysis Cells for Diverse Research Applications
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
Related Articles
- Understanding Electrodes and Electrochemical Cells
- Understanding Flat Corrosion Electrolytic Cells: Applications, Mechanisms, and Prevention Techniques
- Applications of Electrolytic Cells in Purification and Electroplating
- Advanced Techniques in Coating Evaluation Using Electrolytic Cells
- Understanding Electrolytic Cells and Their Role in Copper Purification and Electroplating




















