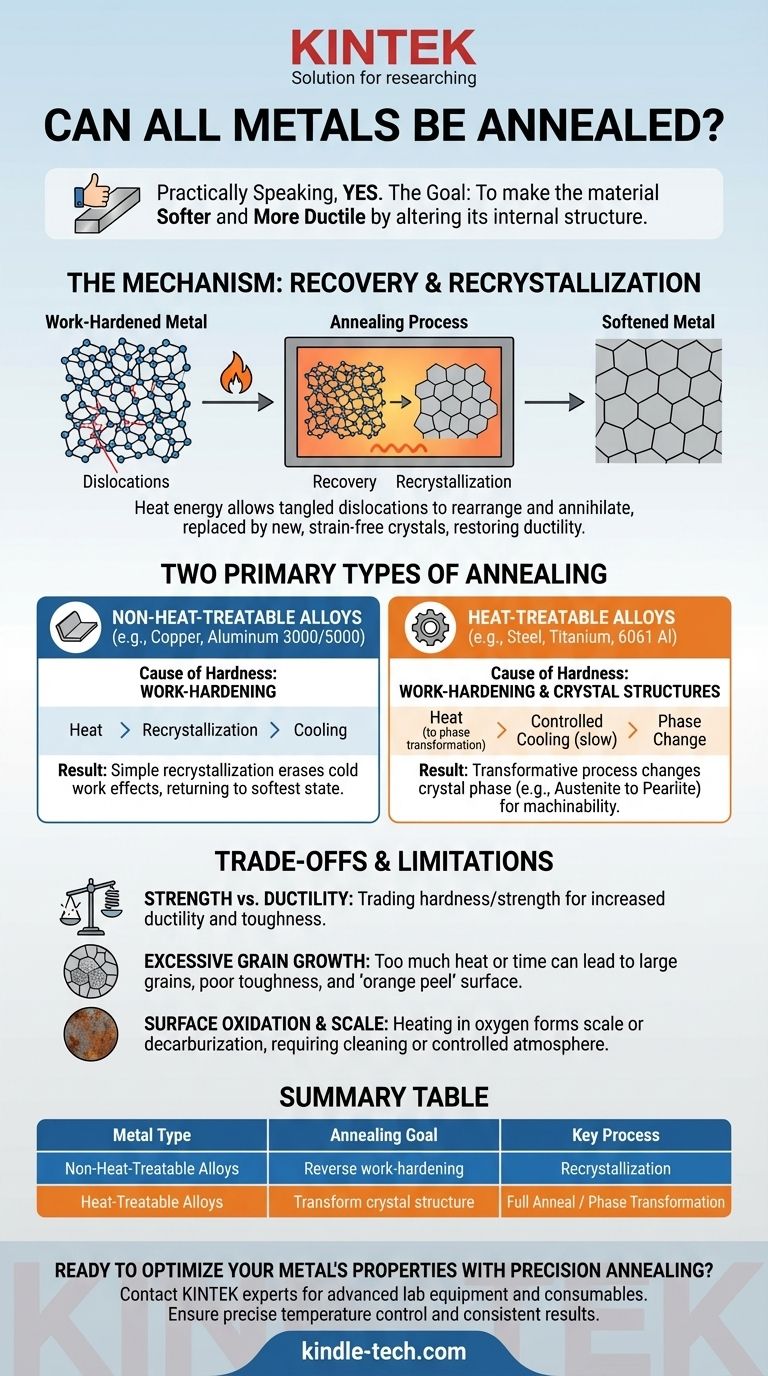
Practically speaking, yes, almost every common metal and alloy can undergo a thermal process called annealing. However, the term "annealing" describes a family of processes, and its specific method and outcome depend entirely on the metal's underlying crystal structure and whether it is heat-treatable. The purpose is always to make the material softer and more ductile by altering its internal structure.
The critical distinction is not if a metal can be heated, but why it is hard in the first place. Annealing effectively reverses hardness from mechanical work (work-hardening), but for high-strength alloys, it involves a more complex transformation of their fundamental crystal structure.
What Annealing Actually Does to a Metal
To understand the scope of annealing, we must first define its core function at a microscopic level. It is a controlled heating and cooling process designed to bring a material closer to its most stable, low-energy state.
Reversing the Effects of Work-Hardening
When you bend, roll, or draw a metal, you are creating microscopic defects in its crystal lattice called dislocations. As these dislocations multiply and tangle, they impede further deformation, making the metal harder, stronger, and more brittle. This is known as work-hardening or strain hardening.
The Mechanism: Recovery and Recrystallization
Annealing reverses this process. By heating the metal to a specific temperature, you give its atoms enough thermal energy to move. This allows the tangled dislocations to rearrange and annihilate, a stage called recovery.
With sufficient heat, entirely new, strain-free crystals (or grains) begin to form and grow, replacing the old, deformed ones. This is recrystallization, which effectively erases the effects of work-hardening and restores the metal's ductility.
The Goal: A Softer, More Ductile State
The result of this process is a metal that is significantly softer and more workable. As the reference states, this reduces the risk of the metal fracturing under stress and makes it far more suitable for subsequent manufacturing steps like machining, stamping, or deep drawing.
Why "Annealing" Varies Between Metal Types
The nuance of the question "Can all metals be annealed?" lies in the fact that there are two primary sources of hardness in metals: work-hardening and heat treatment. The annealing process differs depending on which source of hardness it is intended to remove.
For Non-Heat-Treatable Alloys
This category includes pure metals like copper and aluminum, as well as many of their alloys (e.g., 3000 or 5000 series aluminum). These materials can only be hardened through work-hardening.
For them, annealing is a straightforward recrystallization process. Heating them above their recrystallization temperature simply erases the effects of cold work, returning them to their softest possible state. The process is simple and highly effective.
For Heat-Treatable Alloys
This group includes all carbon and alloy steels, as well as heat-treatable aluminum (e.g., 6061, 7075) and titanium alloys. These materials derive their high strength not just from work-hardening, but from specific, hard crystal structures (like martensite in steel) created by a rapid cooling process (quenching).
For these alloys, annealing is a transformative process. It's not just about removing dislocations; it's about using heat to completely change the crystal phase. For example, a "full anneal" on a hardened steel involves heating it until it transforms into a phase called austenite, then cooling it very slowly to allow a soft, coarse structure of pearlite to form. This makes the ultra-hard steel machinable.
Understanding the Trade-offs and Limitations
While annealing is a powerful tool, it is not without consequences. Its primary purpose is to induce softness, which comes at a direct cost.
The Obvious Trade-off: Strength for Ductility
Annealing makes a metal weaker. You are fundamentally trading hardness and strength for an increase in ductility and toughness. This is the desired outcome when preparing a material for forming, but the finished part will almost certainly require a subsequent heat treatment or work-hardening process to achieve its final required strength.
The Risk of Excessive Grain Growth
If the annealing temperature is too high or held for too long, the newly formed crystals can grow excessively large. While the material will be very soft, large grains can lead to poor toughness and a rough surface finish known as "orange peel" when the part is later formed.
Surface Oxidation and Scale
Heating metals in the presence of oxygen will cause a layer of oxide, or scale, to form on the surface. For some applications, this must be cleaned off. In carbon steels, holding the material at high temperatures for too long can also cause decarburization—the loss of carbon from the surface, which softens the exterior and is often a critical defect. This is why many annealing processes are performed in a controlled, oxygen-free atmosphere.
How to Apply This to Your Project
Your choice of thermal process depends entirely on the material you are using and your ultimate goal for the workpiece.
- If your primary focus is to soften a work-hardened part for further forming (e.g., a copper tube or aluminum sheet): You need a standard recrystallization anneal to restore ductility.
- If your primary focus is to make a high-strength steel part machinable: You need a specific process like a full anneal or spheroidize anneal to transform the microstructure into its softest possible form.
- If your primary focus is to optimize the strength of a heat-treatable alloy: Annealing is just one step in a multi-stage process that will also include solution treating, quenching, and aging.
Understanding these principles allows you to use heat treatment not as a rigid recipe, but as a precise tool to engineer the exact material properties you require.
Summary Table:
| Metal Type | Annealing Goal | Key Process | Result |
|---|---|---|---|
| Non-Heat-Treatable Alloys (e.g., Copper, Aluminum 3000/5000 series) | Reverse work-hardening | Recrystallization | Restores softness and ductility |
| Heat-Treatable Alloys (e.g., Steel, 6061/7075 Aluminum, Titanium) | Transform crystal structure | Full Anneal / Phase Transformation | Creates soft, machinable state |
Ready to optimize your metal's properties with precision annealing? At KINTEK, we specialize in providing advanced lab equipment and consumables tailored to your thermal processing needs. Whether you're working with non-heat-treatable alloys or complex heat-treatable metals, our solutions ensure precise temperature control and consistent results.
Contact our experts today to discuss how we can help you achieve the perfect balance of strength and ductility for your laboratory applications!
Visual Guide
Related Products
- Vacuum Heat Treat Sintering Brazing Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Molybdenum Vacuum Heat Treat Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Graphite Vacuum Furnace High Thermal Conductivity Film Graphitization Furnace
People Also Ask
- How hot should aluminum be for casting? Achieve Perfect Castings with the Right Pouring Temperature
- What is heat treatment as used in metallic materials? Tailor Metal Properties for Superior Performance
- What are the performance advantages of using a Spark Plasma Sintering (SPS) furnace? Enhance CNT Metal Matrix Composites
- Can you solder or braze stainless steel? Yes, with the right preparation and methods.
- Why must high-vacuum annealing furnaces be used for diamond doping? Protect Crystals from Irreversible Graphitization
- What is a vacuum furnace used for? Unlock High-Purity Heat Treatment for Superior Materials
- What is the suitable temperature to process the material in the sintering stage? Find the Perfect Sintering Sweet Spot
- How is heating done in sintering operation? Master the Core Methods for Dense, Strong Parts



















