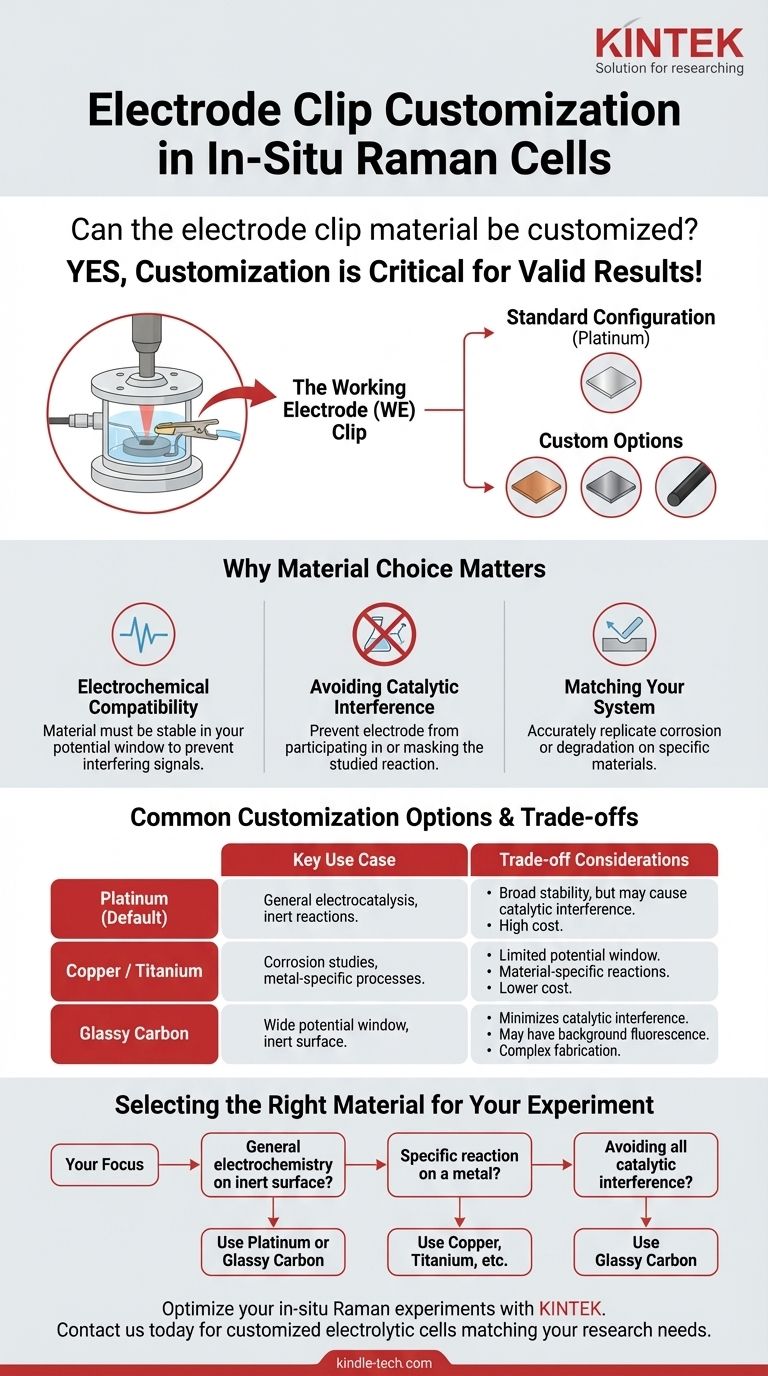
Yes, the material for the electrode clip in an in-situ Raman electrolytic cell can be customized. While the standard configuration is typically a built-in platinum sheet that serves as the working electrode, it is often possible to request replacements made from other materials. Common alternatives include metals like copper and titanium, or non-metals such as glassy carbon.
The ability to customize the working electrode material is not a minor feature—it is a critical requirement for ensuring the validity of your experiment. Your choice of material directly dictates the electrochemical environment and prevents the electrode itself from interfering with the reaction you intend to study.

The Role of the Working Electrode Clip
The Standard Configuration
In a typical three-electrode in-situ Raman cell, the setup is designed for general-purpose electrochemistry. The "clip" is not just a holder; it is the working electrode (WE) where your primary electrochemical reaction occurs.
The standard setup usually includes:
- Working Electrode: A miniature platinum sheet electrode clip.
- Counter Electrode: A platinum wire ring.
- Reference Electrode: An Ag/AgCl electrode.
Why It's More Than Just a Clip
This integrated design simplifies experiments by combining the sample holder and the working electrode into one component. However, this means the material of the clip is an active participant in your experiment, not a passive observer.
Why Material Choice is Critical
The default platinum clip is chosen for its general stability and inertness across a wide range of experiments. However, "general purpose" is not always sufficient for specialized research, where the choice of working electrode material is fundamental.
Ensuring Electrochemical Compatibility
The electrode material must be electrochemically stable within the potential window of your experiment. If the electrode itself oxidizes or reduces under your experimental conditions, it will generate confounding signals and interfere with your measurements.
Avoiding Catalytic Interference
Many electrode materials, especially platinum, are highly catalytic. If you are studying a specific catalytic process, using a platinum clip could introduce unwanted side reactions or mask the true activity of your sample, leading to incorrect conclusions.
Matching Your System of Interest
In many cases, the goal of the experiment is to study a process occurring on a specific material. For example, if you are investigating corrosion on a copper surface or battery anode degradation on titanium, the working electrode clip must be made of that material to accurately replicate the system.
Common Customization Options
Platinum (The Default)
Platinum is the standard for a reason. It is relatively inert in many electrolytes and offers a good potential window for a variety of oxidation and reduction studies. It is the workhorse for general electrocatalysis.
Copper or Titanium
Requesting a clip made of copper or titanium is common when the research focuses on processes unique to these metals. This could include studies on corrosion, electrodeposition, or their specific catalytic properties.
Glassy Carbon
A glassy carbon electrode is an excellent choice when you need a highly inert surface with a very wide potential window, often exceeding that of platinum. It is ideal for experiments where you must be certain that the electrode itself is not participating in or catalyzing the reaction.
Understanding the Trade-offs
Choosing a custom material is not without considerations. You must weigh the benefits against potential drawbacks to make an informed decision.
Potential Window Limitations
While platinum is broadly stable, a material like copper is not. It will readily oxidize at positive potentials, severely limiting the experimental window. You must match the material's stability to your reaction conditions.
Cost and Fabrication
Platinum is expensive. Custom-fabricating clips from other exotic materials can also involve significant cost and lead time. In contrast, common materials like copper are much more affordable.
Raman Signal Interference
The electrode material itself can have a Raman signature or, more commonly, contribute to background fluorescence. This is a critical consideration that must be evaluated for your specific material and laser wavelength.
Selecting the Right Material for Your Experiment
Your choice should be driven entirely by the scientific question you are asking.
- If your primary focus is general electrochemistry on an inert surface: Sticking with the default platinum clip or considering a glassy carbon alternative is your best approach.
- If your primary focus is studying a reaction on a specific metal: You must use a clip customized to that material, such as copper or titanium, to ensure your model is accurate.
- If your primary focus is avoiding all potential catalytic interference from the electrode: A glassy carbon clip is often the safest and most truly "inert" choice available.
Ultimately, aligning the electrode material with your experimental goals is the foundation for obtaining clean, relevant, and publishable in-situ Raman data.
Summary Table:
| Customization Option | Key Use Case | Key Consideration |
|---|---|---|
| Platinum (Default) | General electrocatalysis, inert reactions | Broad stability, but may cause catalytic interference |
| Copper/Titanium | Corrosion studies, metal-specific processes | Limited potential window, material-specific reactions |
| Glassy Carbon | Wide potential window, inert surface | Minimizes catalytic interference, may have background fluorescence |
Optimize your in-situ Raman experiments with KINTEK. The right electrode clip material is crucial for valid, publishable results. Our team specializes in customizing electrolytic cells to match your specific research needs—whether you require platinum, copper, titanium, glassy carbon, or other materials. Contact us today to discuss your experimental requirements and ensure your setup delivers accurate, interference-free data.
Visual Guide
Related Products
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell for Coating Evaluation
- Electrolytic Electrochemical Cell with Five-Port
- Thin-Layer Spectral Electrolysis Electrochemical Cell
- H Type Electrolytic Cell Triple Electrochemical Cell
People Also Ask
- How does anodic oxidation equipment facilitate TiO2 nanotube growth? Precision Control for Advanced Titanium Alloys
- How does electrolytic etching equipment help in identifying the microstructural phases of super duplex stainless steel?
- What is the primary function of a high-precision electrochemical workstation? Optimize Your Reactor Performance
- What is the core function of a laboratory-scale single-chamber circulating electrolytic reactor? Optimize Al Recovery
- What is the necessity of a constant-temperature electrochemical testing system? Ensure Precision in Perovskite Research
- Which performance indicators are measured using a three-electrode electrolytic cell? Evaluate Photocatalysts with KINTEK
- What factors should be considered when selecting an ion-exchange membrane? Balance Selectivity & Conductivity for Your Lab
- What are the standard aperture specifications for the non-sealed and sealed electrolytic cells? Choose the Right Setup for Your Experiment



















