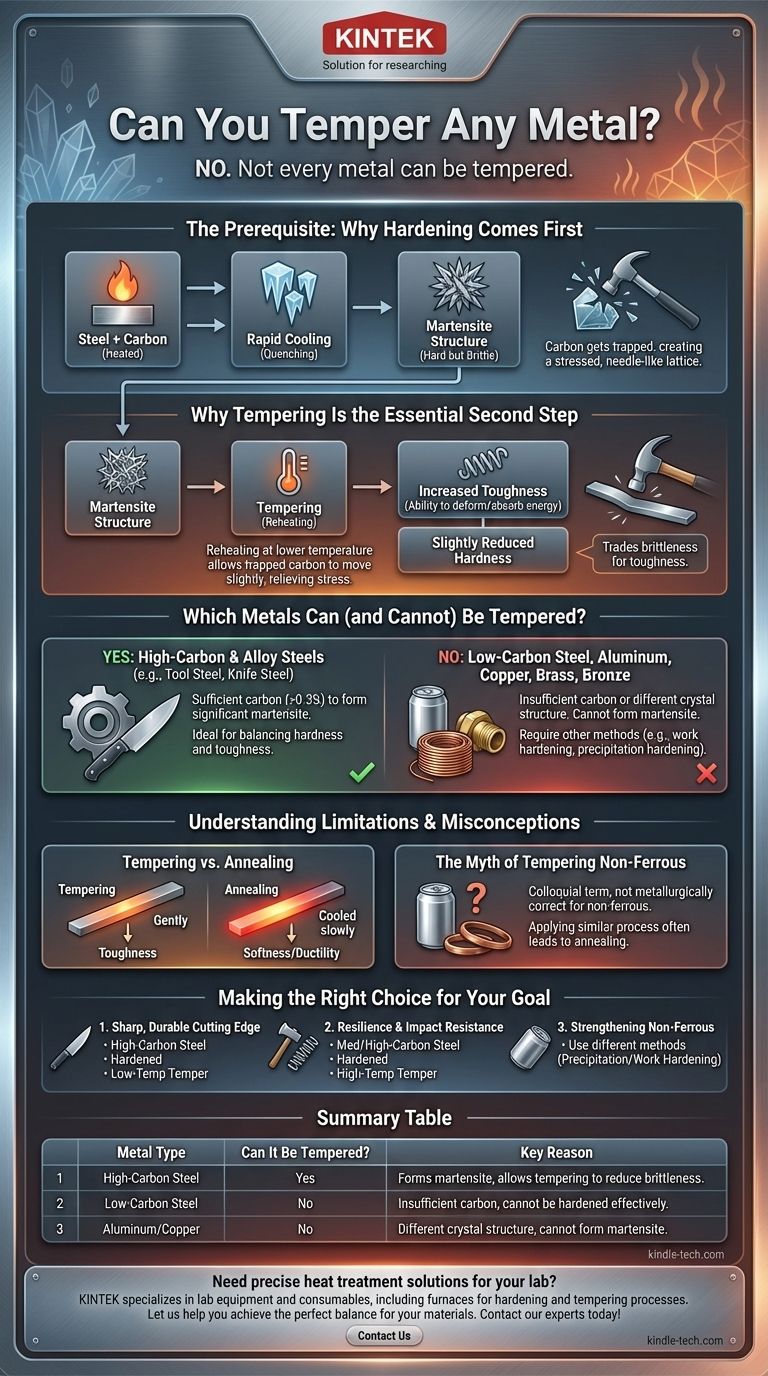
No, not every metal can be tempered. Tempering is a very specific heat treatment designed to reduce the brittleness of a metal that has already been hardened. This process is almost exclusively applied to ferrous alloys, like carbon steel, because their unique crystalline structure is what allows for the initial hardening that makes tempering both possible and necessary.
The ability to temper a metal is not an independent property; it is a corrective step that entirely depends on whether the metal can first be hardened by quenching. If a metal cannot be hardened to form a brittle, martensitic structure, there is nothing to temper.

The Prerequisite: Why Hardening Comes First
Tempering only makes sense when you understand its relationship to hardening. The two processes are two sides of the same coin, used to achieve a precise balance of mechanical properties in steel.
What Is Hardening?
Hardening involves heating steel to a critical temperature and then cooling it very rapidly, a process known as quenching. This rapid cooling traps the metal's internal structure in a highly stressed, disorganized state.
The Role of Carbon in Steel
The key ingredient for this process is carbon. When steel is heated, its iron atoms arrange into a crystal structure that can easily dissolve carbon atoms. Think of it as a loose, open lattice.
Creating the Brittle "Martensite" Structure
Upon quenching, the iron atoms try to snap back into a more compact, room-temperature structure. However, the carbon atoms get trapped, distorting and stressing the lattice. This new, needle-like structure is called martensite, which is extremely hard but also very brittle, like glass.
Why Tempering Is the Essential Second Step
A piece of steel that has only been hardened is often too brittle for practical use. A hardened knife edge would chip, and a hardened hammer would shatter on impact. Tempering solves this problem.
The Problem with Pure Hardness
The martensitic structure created by quenching is strong but has very little "give." Any sharp impact can cause it to fracture catastrophically. This property is known as low toughness.
How Tempering Works
Tempering involves reheating the hardened steel to a much lower, precisely controlled temperature (well below the initial hardening temperature). This gentle heat gives the trapped carbon atoms just enough energy to move slightly and relieve some of the internal stress.
Trading Brittleness for Toughness
This process reduces the overall hardness slightly but dramatically increases the toughness—the metal's ability to deform and absorb energy without fracturing. The final properties are determined by the exact temperature and duration of the tempering process.
Which Metals Can (and Cannot) Be Tempered?
The ability to form martensite is the dividing line. This property is almost exclusive to iron alloys with sufficient carbon.
The Prime Candidates: High-Carbon and Alloy Steels
Steels with a significant carbon content (generally above 0.3%) are the ideal candidates for hardening and tempering. This includes tool steels, spring steels, and many knife steels, where a precise balance of hardness and toughness is critical.
Why Low-Carbon Steel Doesn't Respond
Mild or low-carbon steel simply doesn't have enough carbon to form a significant amount of martensite when quenched. Therefore, it cannot be meaningfully hardened, and since there is no extreme brittleness to correct, tempering has no effect.
Why Metals Like Aluminum and Copper Are Different
Non-ferrous metals like aluminum, copper, brass, and bronze have entirely different crystal structures. They cannot form martensite. They are strengthened through completely different mechanisms, such as work hardening (bending or hammering) or precipitation hardening (an aging process).
Understanding the Limitations and Misconceptions
Confusing different heat treatments is a common pitfall. Clarity on the purpose of each process is crucial for achieving the desired outcome.
Tempering vs. Annealing
Tempering follows hardening to increase toughness. Annealing is a separate process where a metal is heated and cooled very slowly to achieve maximum softness, ductility, and to remove internal stresses. You anneal a metal to make it easy to work with, while you temper it to make it durable in its final form.
The Myth of Tempering Non-Ferrous Metals
While the term "tempering" is sometimes used colloquially for other processes, it is metallurgically incorrect. The mechanism of relieving stress in hardened steel is unique. Applying a similar process to aluminum, for example, would likely result in annealing (softening) it.
Precision is Non-Negotiable
The final balance of hardness and toughness is dictated by the tempering temperature. A difference of even 25°C (approx. 50°F) can produce a measurably different result, which is why industrial processes rely on calibrated ovens, not color charts alone.
Making the Right Choice for Your Goal
Understanding this principle allows you to select the correct material and process for your specific application.
- If your primary focus is creating a sharp, durable cutting edge (e.g., a knife or chisel): You need a high-carbon steel that can be hardened for wear resistance and then tempered at a low temperature to retain most of that hardness while gaining essential toughness.
- If your primary focus is resilience and impact resistance (e.g., a spring, axe, or hammer): You need a medium-to-high-carbon steel that is tempered at a higher temperature, sacrificing significant hardness for maximum toughness.
- If your primary focus is strengthening a non-ferrous metal like aluminum: You must use entirely different methods, such as precipitation hardening (for specific alloys) or work hardening, as quenching and tempering will not work.
Ultimately, mastering a material begins with understanding its fundamental properties and respecting the specific processes required to unlock its potential.
Summary Table:
| Metal Type | Can It Be Tempered? | Key Reason |
|---|---|---|
| High-Carbon Steel | Yes | Forms martensite when quenched, allowing tempering to reduce brittleness. |
| Low-Carbon Steel | No | Insufficient carbon to form martensite; cannot be hardened effectively. |
| Aluminum/Copper | No | Crystal structure cannot form martensite; requires other strengthening methods. |
Need precise heat treatment solutions for your lab? KINTEK specializes in lab equipment and consumables, including furnaces for hardening and tempering processes. Let us help you achieve the perfect balance of hardness and toughness for your materials. Contact our experts today to discuss your laboratory needs!
Visual Guide
Related Products
- 1800℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What temperature does a sealed quench furnace run at? A Guide to Optimizing Your Heat Treatment
- What is the role of a laboratory high-temperature resistance furnace in TSR testing? Quantifying Material Durability
- How do you maintain a hot zone vacuum furnace? Extend Lifespan and Ensure Process Quality
- Why is 1177 °C precision critical for GH3535 furnace treatment? Ensure Microstructural Integrity
- What is the effect of temperature on sintering? Master the Thermal Profile for Superior Results
- What is the process of vacuum melting? Achieve Ultra-Pure Metals for Critical Applications
- What energy transfer happens in a furnace? Master Convection, Conduction & Radiation for Your Process
- What are the benefits of using HIP equipment for high-entropy alloys? Achieve Near-Theoretical Density & Durability



















