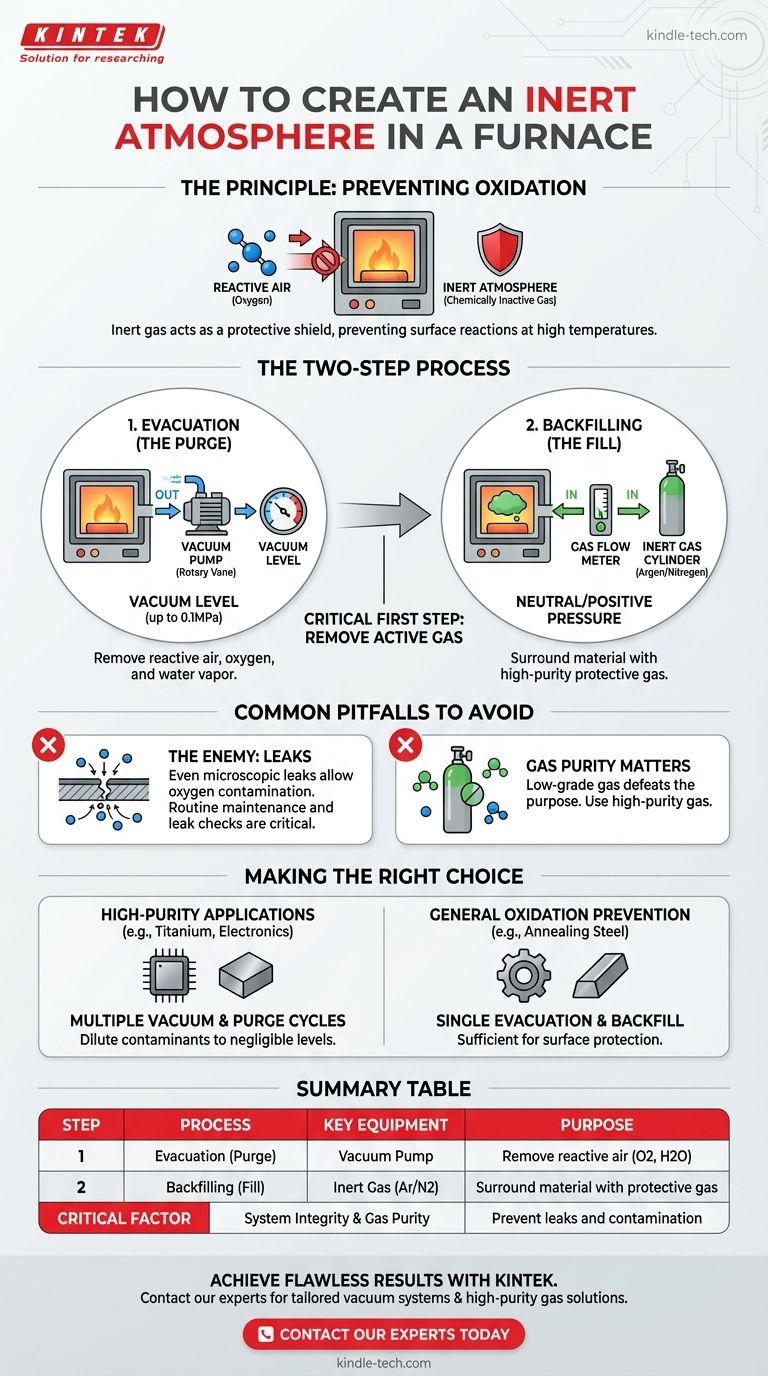
To create an inert atmosphere in a furnace, you first use a vacuum pump to remove the reactive air from the chamber. Once a sufficient vacuum is achieved, the chamber is backfilled, or "purged," with a chemically inactive gas like Argon or Nitrogen, which surrounds the material and prevents it from reacting with any residual oxygen during heat treatment.
The fundamental strategy is not just to add an inert gas, but to first remove the active one. Creating a vacuum is the critical first step that makes the subsequent inert gas purge effective, ensuring a truly non-reactive environment for your material.

The Principle of an Inert Atmosphere
What "Inert" Means in This Context
An inert atmosphere is a chemically inactive environment. The goal is to fill the furnace with a gas that will not react with the materials being heated.
This is critical because at high temperatures, materials like metals become highly susceptible to reacting with oxygen in the air.
The Problem: Preventing Oxidation
The primary purpose of an inert atmosphere is to prevent oxidation and other unwanted surface reactions.
When a material oxidizes, its surface properties change, which can compromise its structural integrity, conductivity, or appearance. The inert gas acts as a protective shield.
The Two-Step Process for Creating the Atmosphere
Step 1: Evacuation (The Purge)
The process begins by sealing the furnace chamber and using a vacuum pump, often a rotary vane type, to remove the ambient air.
This step is essential because it physically removes the vast majority of reactive gases, primarily oxygen and water vapor, from the chamber. A pressure gauge is used to monitor the vacuum level, often aiming for pressures up to 0.1MPa.
Step 2: Backfilling (The Fill)
Once the desired vacuum is reached, the vacuum valve is closed, and an inlet valve is opened to allow a high-purity inert gas, like Argon or Nitrogen, to flow into the chamber.
A gas flow meter and needle valves are used to carefully control the rate at which the gas enters, bringing the chamber back to a neutral or slightly positive pressure. This ensures the material is completely surrounded by the protective gas.
Common Pitfalls to Avoid
The Primary Enemy: Leaks
The most common point of failure in maintaining an inert atmosphere is a leak in the furnace system. Even a microscopic leak can allow oxygen from the outside air to seep in, contaminating the environment.
Routine maintenance is not optional. Regularly inspecting seals, gaskets, and fittings for wear and tear is critical to ensuring the integrity of your atmosphere. Thorough leak detection methods may be required for high-purity applications.
Gas Purity Matters
The inert gas you use must be of high purity. Using a low-grade gas cylinder that contains moisture or oxygen will defeat the entire purpose of the process, as you are introducing contaminants directly into your "inert" environment.
Making the Right Choice for Your Process
The rigor of your process depends entirely on the sensitivity of your material.
- If your primary focus is high-purity applications (e.g., titanium or sensitive electronics): Perform multiple vacuum and purge cycles to dilute any residual contaminants to negligible levels.
- If your primary focus is general oxidation prevention (e.g., annealing steel): A single, well-executed evacuation and backfill cycle is typically sufficient to protect the material's surface.
- If your primary focus is process reliability and repeatability: Make preventative maintenance and system leak checks a mandatory part of your operational checklist.
Ultimately, controlling the furnace atmosphere gives you direct control over your material's final properties.
Summary Table:
| Step | Process | Key Equipment | Purpose |
|---|---|---|---|
| 1 | Evacuation (Purge) | Vacuum Pump | Remove reactive air (oxygen, water vapor) |
| 2 | Backfilling (Fill) | Inert Gas (Argon/Nitrogen) | Surround material with protective gas |
| - | Critical Factor | System Integrity & Gas Purity | Prevent leaks and contamination |
Achieve flawless heat treatment results with a perfectly controlled furnace atmosphere. KINTEK specializes in laboratory furnaces, vacuum systems, and high-purity gas solutions designed for reliable, leak-free performance. Whether you are working with sensitive electronics or general metals, our expertise ensures your materials are protected from oxidation. Contact our experts today to discuss your specific application and receive a tailored solution.
Visual Guide
Related Products
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- Vacuum Heat Treat and Sintering Furnace with 9MPa Air Pressure
People Also Ask
- What is meant by inert atmosphere? A Guide to Preventing Oxidation & Ensuring Safety
- How does an atmosphere furnace facilitate the post-treatment of nickel-plated carbon fibers? Ensure Peak Bonding
- Why nitrogen is used in furnace? A Cost-Effective Shield for High-Temperature Processes
- What is the purpose of inert atmosphere? A Guide to Protecting Your Materials and Processes
- How we can develop inert atmosphere for a chemical reaction? Master Precise Atmospheric Control for Your Lab



















