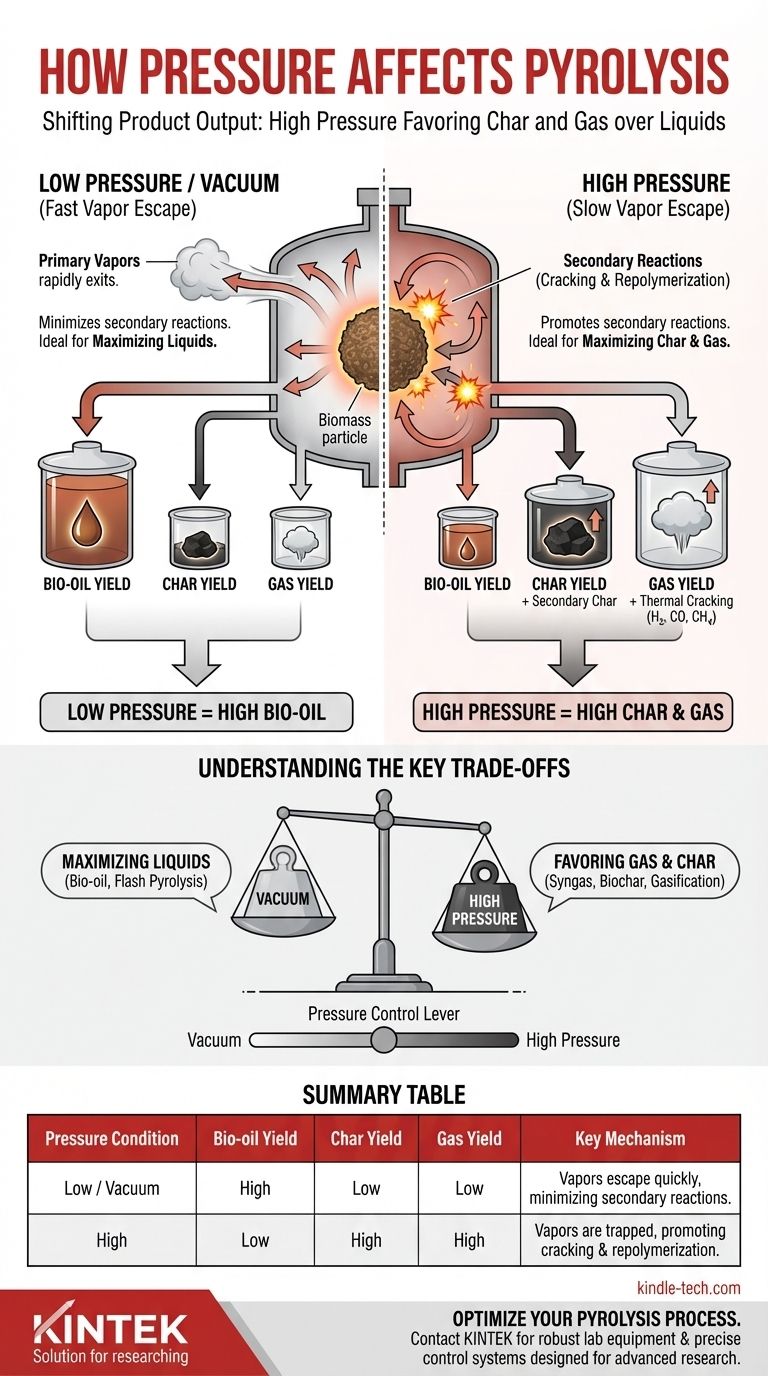
In short, increasing pressure during pyrolysis fundamentally shifts the product output away from liquids and towards more char and gas. This occurs because higher pressure physically hinders the escape of volatile compounds from the solid biomass, forcing them to spend more time in the hot reaction zone where they undergo secondary reactions.
Pressure is a primary control lever in pyrolysis. It directly dictates the residence time of volatile vapors, determining whether they escape to become bio-oil or are converted into secondary char and non-condensable gases.

The Core Mechanism: How Pressure Alters Pyrolysis Pathways
To control a pyrolysis process, you must understand how pressure alters the fundamental physics and chemistry inside the reactor. The primary influence is on the movement of molecules.
Impact on Mass Transfer and Residence Time
At low pressure or in a vacuum, there is a strong driving force for volatile compounds (vapors) to escape the biomass particles as they form. They are quickly pulled away from the hot solid surface.
At high pressure, the surrounding atmosphere pushes back on these escaping vapors. This significantly slows their rate of diffusion out of the particle and the reactor, dramatically increasing their residence time in the hot zone.
Promotion of Secondary Reactions
This increased residence time is the root cause of all subsequent product changes. The primary vapors, now trapped near the hot char surface, are subjected to further thermal decomposition.
These secondary reactions follow two main pathways:
- Cracking: Vapors break down into smaller, thermally stable gas molecules like CO, H₂, and CH₄.
- Repolymerization: Vapors react with each other and the char surface, re-condensing into a more stable, carbon-rich solid known as secondary char.
The Effect of Pressure on Pyrolysis Products
By controlling secondary reactions, pressure directly determines the final yield of liquids, solids, and gases.
Decreased Bio-oil (Liquid) Yield
Bio-oil is produced by rapidly cooling and condensing the primary pyrolysis vapors.
Because high pressure promotes the conversion of these primary vapors into gas and char, fewer of them are left to exit the reactor and be condensed. Consequently, increasing pressure systematically decreases bio-oil yield.
Increased Char Yield
The char produced in pyrolysis comes from both the initial solid biomass (primary char) and the repolymerization of vapors (secondary char).
High pressure is a direct promoter of secondary char formation. This leads to a higher overall solid yield and can alter the char's properties, often making it denser.
Increased Gas Yield
The thermal cracking of trapped vapors into non-condensable gases means that high-pressure pyrolysis will always produce a higher volume of syngas.
This is a key principle used in related processes like gasification, which often operate at elevated pressures specifically to maximize gas production.
Understanding the Key Trade-offs
Choosing an operating pressure is not about right or wrong; it's about optimizing for a specific product. The pressure you choose represents a fundamental trade-off between liquid products and solid/gas products.
Vacuum Pyrolysis: Maximizing Liquids
Operating under a vacuum (negative pressure) creates the ideal conditions for maximizing bio-oil yield.
The vacuum actively pulls vapors out of the reactor as soon as they form, minimizing their residence time and suppressing secondary reactions. This is the principle behind "flash pyrolysis" for biofuel production.
High-Pressure Pyrolysis: Favoring Gas and Char
Elevated pressure is intentionally used when the goal is to produce syngas or a high yield of biochar.
For example, a process focused on hydrogen production would leverage high pressure to maximize the cracking of vapors. A process designed to sequester carbon as biochar would use pressure to encourage secondary char formation.
The Influence of Feedstock
The physical structure of your feedstock matters. A highly porous material like straw allows volatiles to escape more easily than a dense, non-porous material like a plastic polymer.
The effects of pressure will therefore be more pronounced with denser feedstocks where mass transfer is already limited.
Choosing the Right Pressure for Your Goal
Your choice of operating pressure should be a direct reflection of your desired outcome.
- If your primary focus is maximizing bio-oil yield: Operate at a vacuum or as close to atmospheric pressure as possible to rapidly remove vapors and prevent secondary reactions.
- If your primary focus is maximizing syngas production: Use elevated pressures to increase vapor residence time and promote thermal cracking into non-condensable gases.
- If your primary focus is maximizing biochar yield: Employ moderate to high pressures to encourage the repolymerization of vapors into valuable secondary char on the solid surface.
Ultimately, pressure is one of the most powerful tools you have for directing the chemical pathways of pyrolysis toward your intended product.
Summary Table:
| Pressure Condition | Bio-oil Yield | Char Yield | Gas Yield | Key Mechanism |
|---|---|---|---|---|
| Low / Vacuum | High | Low | Low | Vapors escape quickly, minimizing secondary reactions. |
| High | Low | High | High | Vapors are trapped, promoting cracking & repolymerization. |
Ready to optimize your pyrolysis process for maximum yield?
At KINTEK, we specialize in providing robust lab equipment and consumables tailored for advanced pyrolysis research and development. Whether your goal is to maximize bio-oil, syngas, or biochar production, our reactors and systems are designed to give you precise control over key parameters like pressure.
We help you:
- Achieve precise temperature and pressure control for reproducible results.
- Select the right equipment configuration for your specific feedstock and target products.
- Scale your process from lab to pilot with reliable, high-performance systems.
Contact us today to discuss your application and discover how KINTEK can enhance your pyrolysis outcomes. Get in touch via our contact form – let's build your ideal solution together.
Visual Guide
Related Products
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
- Stainless High Pressure Autoclave Reactor Laboratory Pressure Reactor
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
People Also Ask
- What is the difference between electric arc furnace and plasma arc furnace? Choose the Right Tool for Your Heat Processing Needs
- What are the requirements for polymer foam templates for reticulated MAX phase ceramics? Ensure Structural Integrity
- What are the different biomass conversion techniques? Match Feedstock to End Product for Optimal Bioenergy
- What is the thickness of thin films? Unlocking Function from Nanometers to Microns
- Where is soldering commonly used? From Everyday Electronics to Industrial Applications
- What is the most common form of heat treatment? Mastering Annealing, Hardening, and Tempering
- What role does an open reactor play in the SHS process? Enhance Your Surface Coatings Today
- How does heat treating affect the strength of a metal? A Guide to Tailoring Metal Properties



















