At its core, quenching is controlled chemical chaos. In materials science, it is the process of rapidly cooling a hot workpiece, most often steel, to lock in a desirable but unstable atomic structure. Instead of allowing atoms the time to settle into a soft, relaxed state, quenching traps them in a high-energy, strained configuration, dramatically increasing the material's hardness and strength.
Quenching is not just about making something cold; it's a race against time at the atomic level. The goal is to cool a metal so quickly that its atoms cannot rearrange into their preferred, soft crystal structure, instead freezing them in a highly stressed, hard, and brittle state known as martensite.

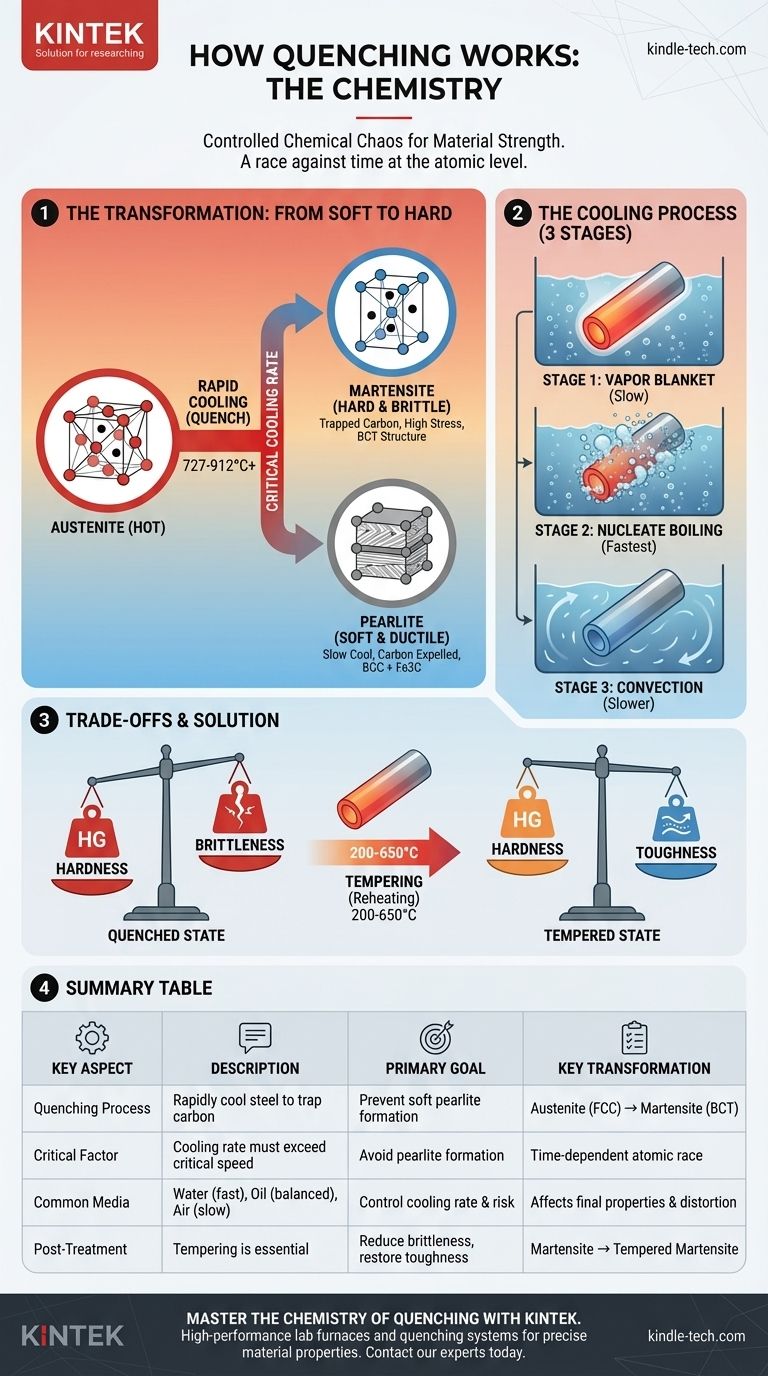
The Goal: From Soft Austenite to Hard Martensite
To understand quenching, you must first understand the different structures, or phases, that steel can adopt. The rate of cooling is the switch that determines which phase becomes dominant.
The High-Temperature State: Austenite
When steel is heated above a critical temperature (typically 727-912°C or 1340-1674°F), it transforms into a phase called austenite. In this state, the iron atoms form a face-centered cubic (FCC) lattice, which has a unique ability to dissolve carbon atoms within its structure. This homogenous, solid solution is the necessary starting point for quenching.
The Slow-Cool Result: Pearlite
If you were to cool the austenitic steel slowly, the iron atoms have ample time to rearrange. They shift from the FCC structure to a more stable body-centered cubic (BCC) structure called ferrite.
Carbon does not dissolve well in ferrite. As a result, the carbon atoms are expelled and combine with iron to form layers of a very hard compound called cementite (iron carbide). This layered structure of ferrite and cementite is known as pearlite, which is relatively soft and ductile.
The Fast-Cool Transformation: Martensite
Quenching short-circuits this natural process. By plunging the hot steel into a medium like water or oil, the cooling is so rapid that the carbon atoms are given no time to escape.
They become trapped within the iron lattice as it attempts to shift to its BCC state. This entrapment of carbon atoms distorts the crystal lattice, forcing it into a highly strained, body-centered tetragonal (BCT) structure called martensite. This immense internal stress is precisely what makes martensite incredibly hard and brittle, as it resists the atomic-level slippage that allows for deformation.
The Cooling Rate: How Quench Media Work
The effectiveness of a quench is defined by its ability to extract heat quickly enough to prevent the formation of pearlite. The process typically occurs in three stages when using a liquid medium.
Stage 1: The Vapor Blanket
Immediately upon immersion, the intense heat of the part vaporizes the surrounding liquid, creating an insulating blanket of vapor. This is known as the Leidenfrost effect, and it actually slows down the initial cooling rate. Agitating the part or the quenchant is critical to break up this barrier.
Stage 2: Nucleate Boiling
As the surface cools slightly, the vapor blanket collapses, and violent boiling begins. This is the fastest stage of heat transfer, where the immense energy of vaporization rapidly pulls heat from the workpiece. This is the stage where the "race" to form martensite is won or lost.
Stage 3: Convection
Once the surface of the part cools below the boiling point of the liquid, boiling ceases. Heat is then removed at a much slower rate through simple convection, as the cooler liquid circulates around the part.
Understanding the Trade-offs: Hardness vs. Brittleness
Achieving maximum hardness through quenching is not a free lunch. It comes with significant risks and compromises that must be managed.
The Price of Hardness: Extreme Brittleness
The same internal stress that makes martensite hard also makes it extremely brittle. A fully quenched, untempered piece of steel can be as fragile as glass and may shatter if dropped or subjected to shock. This makes it unsuitable for most practical applications.
The Risk of Cracking and Distortion
The thermal shock of quenching is immense. If a part has both thick and thin sections, they will cool at different rates, creating massive internal stresses. This can cause the part to warp, distort, or even crack during the quenching process itself.
The Solution: Tempering
Because of this brittleness, a quenched part is almost always subjected to a secondary heat treatment called tempering. The part is reheated to a much lower temperature (e.g., 200-650°C or 400-1200°F) and held for a specific time.
This process allows some of the trapped carbon to precipitate and slightly relieves the internal stress in the martensitic structure. Tempering reduces hardness but critically restores a measure of toughness—the ability to absorb energy and deform without fracturing.
Making the Right Choice for Your Goal
The choice of quenchant and process depends entirely on the steel alloy and the desired final properties.
- If your primary focus is maximum hardness in simple carbon steels: A severe water or brine quench is effective, but carries the highest risk of cracking and distortion.
- If your primary focus is balancing hardness and toughness in alloy steels: An oil quench provides a slower cooling rate, mitigating the risk of cracking while still being fast enough to form martensite.
- If your primary focus is minimizing distortion in complex or high-alloy parts: A very slow air quench may be used for specific "air-hardening" tool steels, which contain alloys that slow the transformation to pearlite.
Ultimately, understanding quenching chemistry empowers you to precisely dictate a material's final properties by controlling its journey from one atomic state to another.
Summary Table:
| Key Aspect | Description |
|---|---|
| Primary Goal | Rapidly cool steel to trap carbon atoms, preventing soft pearlite formation. |
| Key Transformation | Austenite (FCC) → Martensite (BCT), a hard, brittle structure. |
| Critical Factor | Cooling rate must exceed the critical speed to avoid pearlite. |
| Common Quench Media | Water (fastest, highest risk), Oil (balanced), Air (slowest, for specific alloys). |
| Post-Quench Treatment | Tempering is essential to reduce brittleness and restore toughness. |
Ready to achieve precise material properties in your lab? The right quenching process is critical for success. KINTEK specializes in high-performance lab furnaces and quenching systems designed for exacting heat treatment protocols. Whether you're working with carbon steels, alloy steels, or complex tool steels, our equipment ensures the controlled cooling rates you need to form the desired martensitic structure reliably and safely.
Let us help you master the chemistry of quenching. Contact our experts today to discuss your specific laboratory requirements and discover how KINTEK's solutions can enhance your research and development outcomes.
Visual Guide
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1800℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- Why is a high-vacuum environment necessary for sintering aluminum composites? Achieve Superior Bonding & Density
- What is the process of a vacuum furnace? Achieve Purity and Precision in High-Temp Processing
- What is the vacuum brazing technique? Achieve Superior, Flux-Free Metal Joining
- Does heat transfer through a vacuum? Discover the Power of Thermal Radiation in Your Lab
- What is the significance of thermal gradient simulation and thermal cycling furnaces? Ensure Reactor Component Safety
- What temperature is required for pyrolysis? Mastering the Key Control for Your Desired Product
- What furnace is used for heat treatment? Match Your Process to the Perfect Equipment
- What is the function of the furnace in the laboratory? A Tool for Precise Thermal Transformation



















