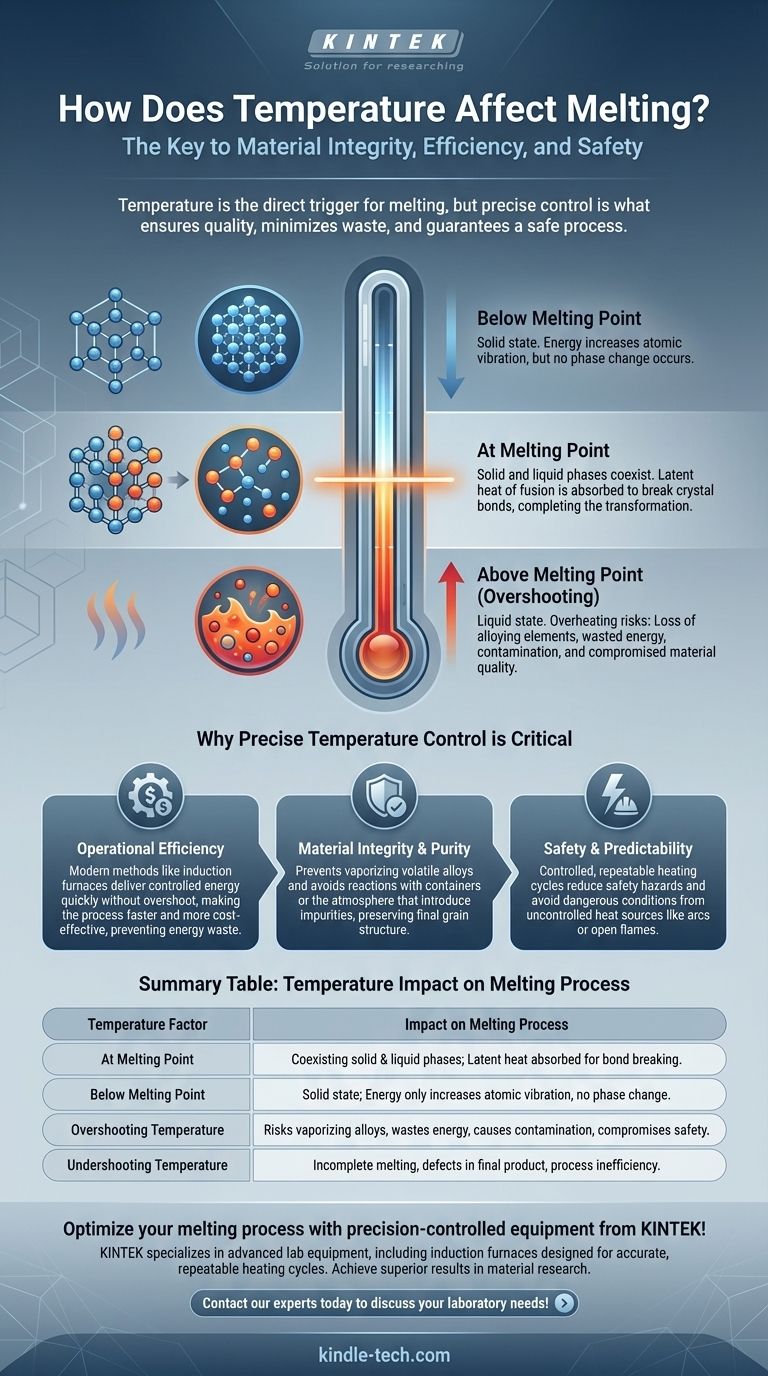
In short, temperature is the direct trigger for melting. For any crystalline solid, such as a metal, to transform into a liquid, it must absorb enough thermal energy to reach a specific threshold known as its melting point. At this temperature, the atoms or molecules gain enough energy to break free from their fixed, ordered structure.
The critical insight is not simply that heat causes melting, but that precise temperature control is the key to preserving material integrity, maximizing efficiency, and ensuring a safe, predictable process. It's the difference between simple transformation and value destruction.

The Mechanics of Melting: Beyond a Simple State Change
Understanding how temperature drives melting reveals why control is so important. The process is more nuanced than simply turning up the heat.
Reaching the Melting Point
Every pure crystalline substance has a distinct melting point. This is the temperature at which the solid and liquid phases can exist in equilibrium.
Below this point, the substance is solid. As you add heat, its temperature rises, and its atoms vibrate more intensely within their crystal lattice.
The Energy of Transformation
Once the material reaches its melting point, its temperature stops rising, even as you continue to add heat.
This additional energy, known as the latent heat of fusion, is used exclusively to break the bonds holding the crystal structure together, completing the transition from solid to liquid.
Why Precise Temperature Control is Critical
Simply melting a material is rarely the end goal. The quality of the final liquid product depends heavily on managing the temperature throughout the process.
Preventing Loss of Alloying Elements
Many materials, especially metals, are alloys—a mixture of different elements. These elements often have different melting and boiling points.
If you overheat the mixture far beyond what is necessary to melt it, you risk vaporizing or oxidizing the more volatile, valuable alloying elements. This fundamentally changes the composition and properties of the final product.
Ensuring Operational Efficiency
Heating a substance beyond its required melting temperature is a direct waste of energy, which increases operational costs.
Modern methods, like the induction furnaces mentioned in process documentation, are efficient precisely because they deliver controlled energy directly to the material. They are designed to reach the target temperature quickly without significant overshoot, making the operation faster and more cost-effective.
Maintaining Material Quality
Excessive heat can introduce other problems. It can cause the molten material to react with the container (crucible) or the atmosphere, introducing impurities.
Furthermore, extreme temperatures can negatively affect the material's final grain structure once it cools and solidifies, potentially compromising its strength or other physical properties.
Understanding the Trade-offs
Achieving the perfect melt involves balancing competing factors. Mismanaging temperature in either direction has clear consequences.
Undershooting the Temperature
The most obvious risk is an incomplete melt. This can result in solid inclusions in a casting, leading to defects and structural failure. It halts the process and requires reheating, wasting time and energy.
Overshooting the Temperature (Overheating)
This is often the more damaging mistake. The consequences include the loss of alloys, wasted energy, increased risk of contamination, and potential damage to equipment.
Extremely high temperatures, especially from uncontrolled sources like arcs or combustion, also introduce significant safety hazards.
Applying This to Your Process
Your specific approach to temperature control should be dictated by your primary objective.
- If your primary focus is material purity and alloy integrity: Heat the material only to the temperature required for a complete, fluid melt and hold it there, avoiding any significant overshoot.
- If your primary focus is energy efficiency and speed: Utilize a heating method, such as induction, that delivers energy directly and precisely, minimizing heat loss to the environment and preventing wasted energy from overheating.
- If your primary focus is safety and predictability: Employ systems that offer controlled, repeatable heating cycles and do not rely on volatile processes like open flames or electric arcs.
Ultimately, mastering the melting process comes down to treating temperature not as a brute-force tool, but as a precision instrument.
Summary Table:
| Temperature Factor | Impact on Melting Process |
|---|---|
| At Melting Point | Solid and liquid phases coexist; latent heat of fusion is absorbed to break bonds. |
| Below Melting Point | Material remains solid; energy increases atomic vibration but no phase change occurs. |
| Overshooting Temperature | Risks vaporizing alloying elements, wastes energy, causes contamination, and compromises safety. |
| Undershooting Temperature | Leads to incomplete melting, defects in final product, and process inefficiency. |
Optimize your melting process with precision-controlled equipment from KINTEK!
Whether you're working with metals, alloys, or other materials, precise temperature management is key to preserving material integrity, maximizing efficiency, and ensuring safety. KINTEK specializes in advanced lab equipment, including induction furnaces designed for accurate, repeatable heating cycles.
Let us help you achieve superior results—contact our experts today to discuss your specific laboratory needs!
Visual Guide
Related Products
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What are the results of sintering? From Powder to High-Strength Solid Parts
- What material is resistant to extreme heat? Match the Right Material to Your Extreme Application
- What is the disadvantage of heat treatment? High Costs, Material Risks, and Operational Complexity
- What role does an industrial Hot Isostatic Pressing (HIP) system play? Mastering ODS Steel Consolidation
- What is the difference between cast and sintered parts? Choose the Right Metal Forming Process
- Why is a high-performance laboratory oven required for constant temperature treatment in mineral kinetic studies?
- What is a hot air oven for a chemistry lab? Master Dry Heat Sterilization & Drying
- What is the composition of pyrolysis liquids? A Deep Dive into Bio-Oil's Chemical Makeup



















