At its core, making steel in an induction furnace is a process of melting metal from the inside out. Unlike traditional furnaces that use external flames or electric arcs, an induction furnace uses a powerful, fluctuating magnetic field to generate intense heat directly within the steel scrap itself. This is achieved by passing a high-frequency alternating current through a copper coil, which induces electrical currents (known as eddy currents) inside the metal, causing it to melt rapidly due to its own electrical resistance.
The central principle of an induction furnace is its contactless heating method. By using electromagnetism to generate heat directly within the steel, it offers exceptional control over temperature and chemical composition, avoiding contamination from external fuel or electrodes.

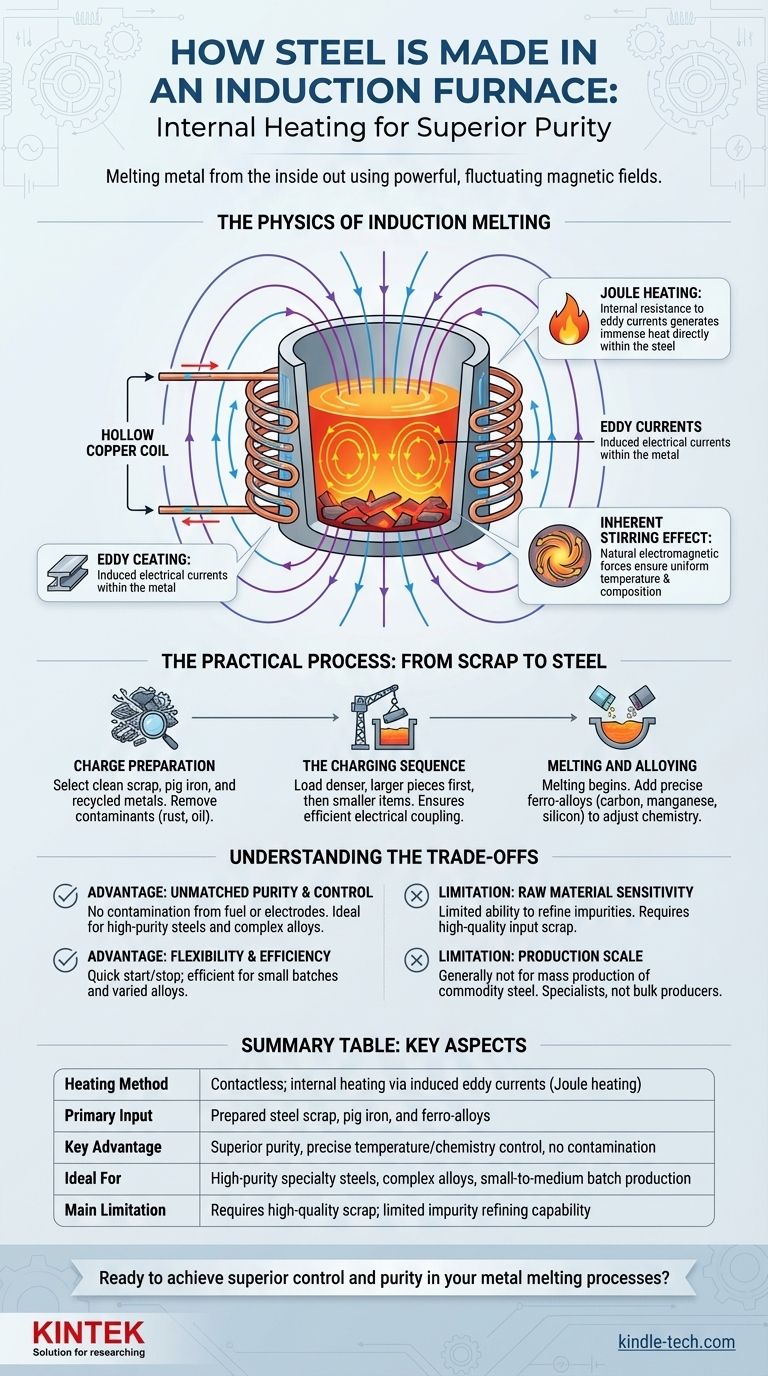
The Physics of Induction Melting
To truly understand the process, you must first grasp the underlying physics. The entire operation hinges on a principle discovered in the 1830s: electromagnetic induction.
Creating the Magnetic Field
The process begins with a high-frequency power supply. This unit sends a powerful alternating current (AC) through a hollow copper coil that encircles a crucible containing the metal charge. The coil itself does not get excessively hot because it is typically cooled with circulating water.
Inducing the Eddy Currents
As the alternating current flows through the coil, it generates a potent and rapidly changing magnetic field around and inside the crucible. This magnetic field penetrates the electrically conductive steel scrap placed within it. This fluctuating field, in turn, induces loops of electrical current within the metal itself, known as eddy currents.
The Power of Joule Heating
The steel, like any conductor, has electrical resistance. As the induced eddy currents flow through this resistance, they generate immense heat in a phenomenon known as Joule heating. It is this internal heat—not an external source—that raises the temperature of the steel past its melting point.
The Inherent Stirring Effect
A secondary benefit of the strong electromagnetic forces is a natural stirring action within the molten metal bath. This constant circulation ensures that the temperature remains uniform throughout the melt and helps alloying elements dissolve completely, leading to a highly consistent and homogenous final product.
The Practical Process: From Scrap to Steel
While the physics are elegant, the practical operation is a carefully managed industrial process that directly impacts the quality of the final steel.
Charge Preparation
The process begins long before the power is switched on. The charge material—typically steel scrap, pig iron, and other recycled metals—must be carefully selected and prepared. It needs to be free from excessive rust, oil, sand, and other non-metallic contaminants, as these can introduce impurities and reduce the furnace's efficiency.
The Charging Sequence
Loading the furnace, or charging, follows a specific protocol. Denser, larger pieces of scrap are loaded first to establish a solid base on the furnace floor. Smaller pieces and turnings are then added to fill the gaps. This ensures good electrical coupling and an efficient start to the melting process.
Melting and Alloying
Once charged, the power is applied, and the melting begins. As the charge collapses into a liquid pool, operators can add precise, pre-weighed amounts of ferro-alloys and other elements like carbon, manganese, and silicon. These additions are what adjust the molten iron's chemistry to meet the exact specifications of the desired steel grade.
Understanding the Trade-offs
No technology is a universal solution. The induction furnace's unique mechanism gives it distinct advantages and clear limitations.
Advantage: Unmatched Purity and Control
Because the heat is generated internally, there is no contamination from combustion by-products (as in a fossil-fuel furnace) or carbon electrodes (as in an Electric Arc Furnace). This makes induction furnaces ideal for producing high-purity steels and complex alloys where precise chemistry is non-negotiable.
Advantage: Flexibility and Efficiency
Induction furnaces can be started and stopped relatively quickly with less energy loss compared to other furnace types that must be held at temperature. This makes them highly efficient for smaller batch sizes and foundries that produce a variety of different alloys throughout the day.
Limitation: Raw Material Sensitivity
The primary drawback is a limited ability to refine out impurities like phosphorus and sulfur. Unlike an Electric Arc Furnace, which can use a slag process to actively remove these elements, an induction furnace largely relies on the cleanliness of the input material. High-quality input scrap is essential for producing high-quality steel.
Limitation: Production Scale
While modern induction furnaces are growing in size and capacity, they are generally not used for the mass production of commodity steel in the same way as multi-hundred-ton Basic Oxygen or Electric Arc Furnaces. They are specialists, not bulk producers.
How to Apply This to Your Goal
The choice of melting technology is dictated entirely by the desired outcome and operational constraints.
- If your primary focus is producing high-purity specialty steels or complex alloys: The precise temperature and chemical control of an induction furnace makes it the superior choice.
- If your primary focus is recycling a wide variety of scrap into standard-grade steel: An Electric Arc Furnace (EAF) is often more economical at scale and more tolerant of lower-quality raw materials.
- If your primary focus is producing massive volumes of steel from raw iron: The Basic Oxygen Furnace (BOF) remains the dominant technology for large, integrated steel mills.
Understanding the principle of internal Joule heating is the key to leveraging the specific advantages of an induction furnace for your application.
Summary Table:
| Key Aspect | Description |
|---|---|
| Heating Method | Contactless; internal heating via induced eddy currents (Joule heating) |
| Primary Input | Prepared steel scrap, pig iron, and ferro-alloys |
| Key Advantage | Superior purity, precise temperature/chemistry control, no contamination |
| Ideal For | High-purity specialty steels, complex alloys, small-to-medium batch production |
| Main Limitation | Requires high-quality scrap; limited impurity refining capability |
Ready to achieve superior control and purity in your metal melting processes?
KINTEK specializes in high-performance lab equipment and consumables for metallurgical research and production. Whether you are developing new alloys or optimizing your melting operations, our solutions are designed to meet the precise demands of laboratory and pilot-scale environments.
Contact our experts today to discuss how our equipment can enhance your steelmaking and materials development workflow.
Visual Guide
Related Products
- Lab-Scale Vacuum Induction Melting Furnace
- Vacuum Heat Treat Furnace and Levitation Induction Melting Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Vertical Laboratory Tube Furnace
People Also Ask
- What are the advantages of induction melting? Achieve Faster, Cleaner, and More Controlled Metal Melting
- What is the vacuum induction method? Master High-Purity Metal Melting for Advanced Alloys
- What is the difference between induction melting and vacuum induction melting? Choosing the Right Process for Purity
- What types of metals are typically processed in a vacuum induction melting furnace? High-Purity Alloys for Critical Applications
- How does induction work in a vacuum? Achieve Ultra-Pure Metal Melting with VIM



















