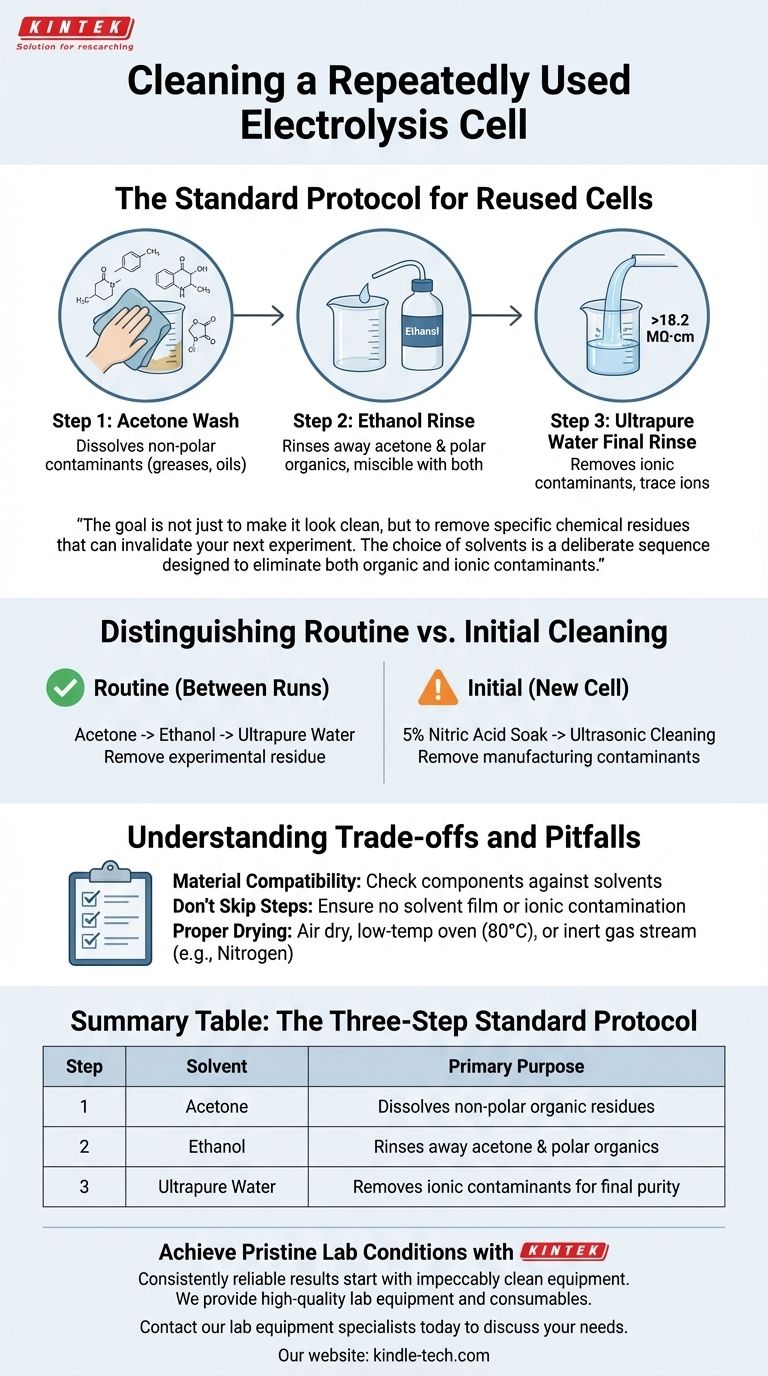
To properly clean a repeatedly used electrolysis cell, the standard procedure involves a three-step solvent wash. You should first wipe the inner walls with acetone, follow with an ethanol rinse, and finish with a final rinse using ultrapure water with a resistivity greater than 18.2 MΩ·cm.
The goal of cleaning an electrochemical cell is not just to make it look clean, but to remove specific chemical residues that can invalidate your next experiment. The choice of solvents—acetone, ethanol, and ultrapure water—is a deliberate sequence designed to eliminate both organic and ionic contaminants.

The Standard Protocol for Reused Cells
Proper cleaning between experiments is critical for achieving reproducible and accurate results. Each step in the following sequence targets a different class of potential contaminants left over from the previous run.
Step 1: Dissolving Organic Residue with Acetone
The first step is to wipe the cell's inner surfaces with acetone. Acetone is a powerful organic solvent effective at dissolving a wide range of non-polar contaminants.
This includes greases, oils, and many organic byproducts that may have formed during the electrolysis process. This initial wipe removes the bulk of the residue.
Step 2: Intermediate Rinsing with Ethanol
After the acetone wipe, rinse the cell thoroughly with ethanol. Ethanol is a polar solvent that is miscible with both acetone and water.
This makes it the perfect intermediate rinse. It washes away any remaining acetone and dissolves other polar organic compounds that acetone may have missed, ensuring no solvent film is left behind.
Step 3: Final Purity Rinse with Ultrapure Water
The final and most critical step is to rinse the cell with ultrapure water. The specified resistivity of >18.2 MΩ·cm signifies Type I ultrapure water.
This water is essentially free of any ions that could adsorb onto the cell walls or electrodes. Using lower-grade water would risk re-contaminating the "clean" cell with trace ions, which can interfere with sensitive electrochemical measurements.
Distinguishing Between Routine and Initial Cleaning
It is crucial to understand that the cleaning protocol for a routinely used cell differs significantly from the initial preparation of a brand-new cell.
The Goal of Routine Cleaning: Removing Experimental Residue
The acetone-ethanol-water sequence is designed for routine cleaning between experiments. Its purpose is to gently but effectively remove the specific reactants and products from the most recent experiment without damaging the cell.
The Goal of Initial Cleaning: Removing Manufacturing Contaminants
A new cell, in contrast, may require a much more aggressive treatment, such as soaking in 5% nitric acid. This process is designed to strip away any metallic residues, machine oils, or other contaminants from the manufacturing and shipping process. This is a one-time commissioning step, not a daily cleaning procedure.
Understanding the Trade-offs and Pitfalls
While the standard protocol is highly effective, awareness of potential issues is key to maintaining both your equipment and your experimental integrity.
Material Compatibility
Always verify that your cell components (including any gaskets, seals, or frits) are chemically compatible with acetone and ethanol. Some plastics and polymers can be damaged by strong organic solvents.
The Risk of Skipping Steps
Each step is essential. Skipping the ethanol rinse can lead to poor removal of acetone, leaving a residue. Rinsing with regular deionized or tap water instead of ultrapure water will almost certainly introduce ionic contamination.
The Importance of Drying
After the final rinse, the cell must be dried properly. You can allow it to air dry, dry it in a low-temperature oven (e.g., 80°C), or use a stream of inert gas like nitrogen. The goal is to remove the water without introducing dust or other airborne contaminants.
Making the Right Choice for Your Goal
Selecting the correct cleaning method is fundamental to the quality of your electrochemical data.
- If your primary focus is running successive experiments in the same cell: Adhere strictly to the three-step acetone, ethanol, and ultrapure water protocol.
- If your primary focus is preparing a brand-new cell for its first use: Use the more aggressive nitric acid soak and ultrasonic cleaning method to remove manufacturing contaminants.
- If you suspect inorganic or metallic contamination in a used cell: A dilute acid wash (weaker than for a new cell) may be necessary, but always follow it with the full ultrapure water rinse protocol.
Ultimately, a disciplined and consistent cleaning regimen is the foundation of reliable electrochemical analysis.
Summary Table:
| Step | Solvent | Primary Purpose |
|---|---|---|
| 1 | Acetone | Dissolves non-polar organic residues (greases, oils) |
| 2 | Ethanol | Rinses away acetone and polar organics |
| 3 | Ultrapure Water (>18.2 MΩ·cm) | Removes ionic contaminants for final purity |
Achieve Pristine Lab Conditions with KINTEK
Consistent, reliable results start with impeccably clean equipment. The precise cleaning protocol detailed above is essential for maintaining the integrity of your electrolysis cells and the accuracy of your electrochemical analysis.
At KINTEK, we specialize in providing the high-quality lab equipment and consumables—including the solvents and ultrapure water systems—that your laboratory needs to execute these critical procedures effectively. Our expertise supports researchers in achieving reproducible data and maintaining peak instrument performance.
Ensure your next experiment is a success. Contact our lab equipment specialists today to discuss your specific needs and how we can help you maintain the highest standards of cleanliness and accuracy in your work.
Visual Guide
Related Products
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- Optical Water Bath Electrolytic Electrochemical Cell
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
People Also Ask
- What are the procedures for after using a double-layer water-bath electrolytic cell? Ensure Equipment Longevity and Data Accuracy
- What are the key features of the five-port water bath electrolytic cell? Precision Control for Electrochemical Experiments
- What are the standard aperture sizes on the lid of the multifunctional electrolytic cell? Key Ports for Your Electrochemical Setup
- What is the overall structure of the H-type double-layer optical water bath electrolytic cell? Precision Design for Controlled Experiments
- When is professional repair required for a double-layer water-bath electrolytic cell? Protect Your Lab's Precision and Safety



















