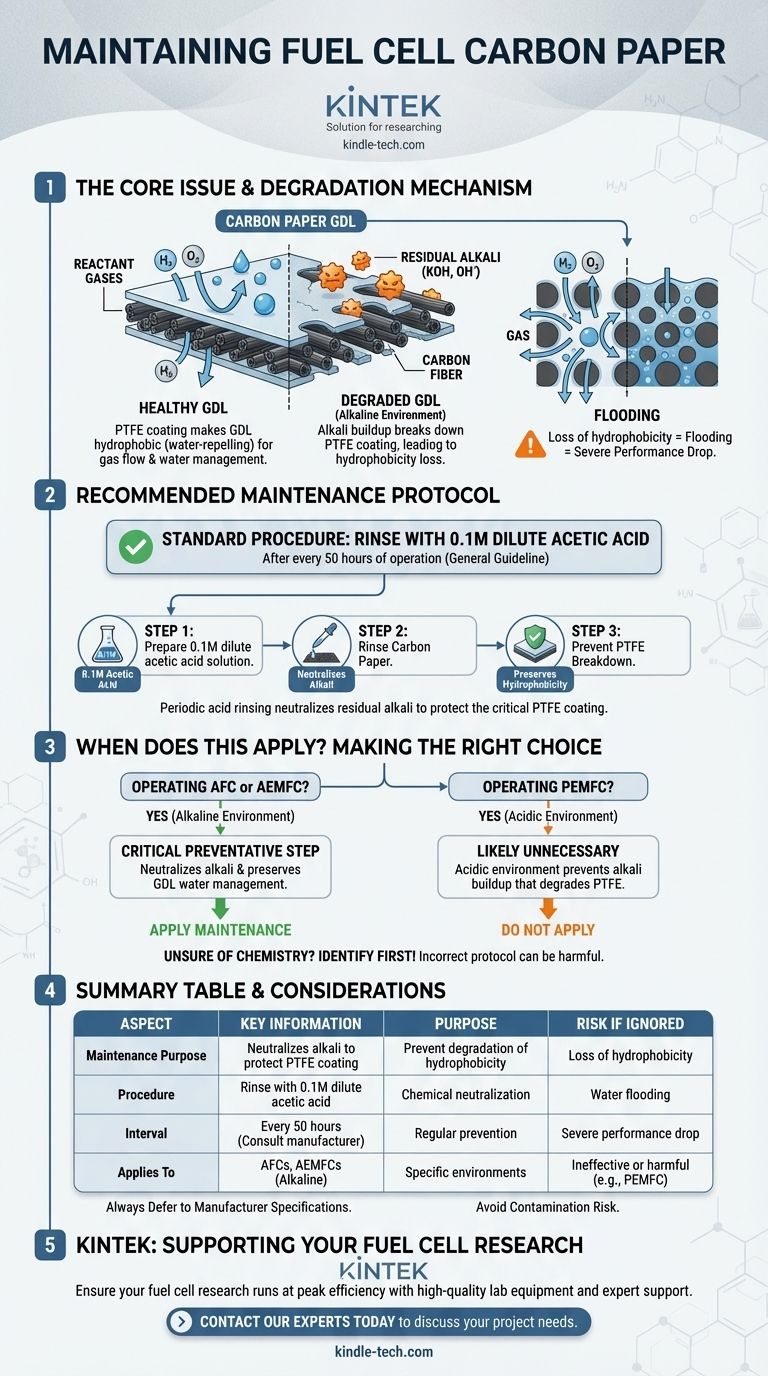
To properly maintain carbon paper from a fuel cell, it must be periodically treated to counteract chemical degradation. The standard procedure is to rinse the carbon paper with a 0.1M dilute acetic acid solution after every 50 hours of operation. This action is specifically designed to neutralize any residual alkali that accumulates, which in turn prevents the breakdown of the paper's critical Polytetrafluoroethylene (PTFE) coating.
The core issue is not the carbon paper itself, but the degradation of its hydrophobic PTFE coating by alkali buildup during operation. Periodic, gentle acid rinsing is a preventative measure to neutralize this alkali, preserving the component's ability to manage water and ensuring consistent fuel cell performance.

The Role of Carbon Paper in a Fuel Cell
To understand the maintenance, you must first understand the function. Carbon paper serves as the Gas Diffusion Layer (GDL), a component with two critical responsibilities.
Function 1: Diffusing Reactant Gases
The primary job of the GDL is to act as a porous pathway. It allows reactant gases, like hydrogen and oxygen, to travel from the flow field channels and distribute evenly across the surface of the catalyst layer, where the electrochemical reactions occur.
Function 2: Managing Water
The GDL's second role is managing water. It must allow product water to exit the cell to prevent "flooding," while also helping to maintain adequate humidity for the proton exchange membrane to function efficiently. This water management is achieved through a special coating.
The Importance of the PTFE Coating
Carbon paper is treated with PTFE (Teflon), which makes it hydrophobic, or water-repelling. This property is essential. It forces liquid water out of the pores, keeping them open for gas to flow through. Without this hydrophobic nature, the paper would become waterlogged, blocking reactants and causing a dramatic drop in fuel cell performance.
Understanding the Degradation Mechanism
The specified maintenance directly targets the chemical breakdown of the PTFE coating. This degradation is most common in specific types of fuel cells.
The Source of Alkali Buildup
This maintenance protocol is specifically relevant for fuel cells that operate in an alkaline environment, such as Alkaline Fuel Cells (AFCs) or Anion Exchange Membrane Fuel Cells (AEMFCs). In these systems, an alkaline electrolyte or environment can leave residual alkali (like potassium hydroxide, KOH) on the GDL.
How Alkali Damages PTFE
Alkaline substances, particularly hydroxide ions (OH⁻), can chemically attack the incredibly stable carbon-fluorine bonds that make up the PTFE polymer. This process slowly breaks down the coating, stripping it from the carbon fibers.
The Consequence: Loss of Hydrophobicity
As the PTFE degrades, the carbon paper loses its water-repelling properties. It becomes hydrophilic (water-attracting), leading to pore blockage by liquid water. This condition, known as flooding, starves the catalyst of fuel and oxidant, causing severe and often irreversible performance loss.
Common Pitfalls and Considerations
While the procedure is simple, its context is critical. Applying it incorrectly can be counterproductive.
This Protocol is Not Universal
This maintenance is highly specific to fuel cells with alkaline environments. It is generally not necessary for more common Proton Exchange Membrane Fuel Cells (PEMFCs), which operate in an acidic environment and do not suffer from alkali buildup. Applying acid in that context would be redundant.
Risk of Contamination
Any maintenance procedure introduces the risk of contamination. Using impure water or acid, or improperly handling the delicate carbon paper, can introduce foreign materials that harm the fuel cell's catalyst or membrane.
Always Defer to Manufacturer Specifications
The 50-hour interval is a general guideline. The exact type of fuel cell, its operating conditions, and the specific materials used will dictate the ideal maintenance schedule. Always consult the documentation provided by your component or system manufacturer first.
Making the Right Choice for Your Goal
Use the following guidelines to decide if this procedure applies to your work.
- If you are operating an Alkaline Fuel Cell (AFC) or AEMFC: This periodic acetic acid rinse is a critical preventative step to neutralize alkali and preserve the GDL's water management capability.
- If you are operating a Proton Exchange Membrane Fuel Cell (PEMFC): This procedure is likely unnecessary, as the acidic operating environment prevents the type of alkali buildup that degrades PTFE.
- If you are unsure of your fuel cell's chemistry: Your first step is to identify it, as applying the wrong maintenance protocol can be ineffective or even harmful to the components.
Ultimately, understanding the underlying chemistry of your system is the key to effective maintenance and reliable performance.
Summary Table:
| Aspect | Key Information |
|---|---|
| Purpose of Maintenance | Neutralizes alkali buildup to prevent degradation of the hydrophobic PTFE coating. |
| Recommended Procedure | Rinse with 0.1M dilute acetic acid solution. |
| Typical Interval | After every 50 hours of operation (consult manufacturer specs). |
| Applies To | Alkaline environment fuel cells (AFCs, AEMFCs). Not for standard PEMFCs. |
| Primary Risk if Ignored | Loss of hydrophobicity, leading to water flooding and severe performance drop. |
Ensure your fuel cell research or operation runs at peak efficiency with the right lab equipment and expert support.
KINTEK specializes in providing high-quality lab equipment and consumables, serving the precise needs of laboratories. Whether you are developing next-generation fuel cells or maintaining critical systems, having reliable components is key.
Contact our experts today to discuss how our solutions can support your fuel cell projects and help you maintain optimal performance.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell Gas Diffusion Liquid Flow Reaction Cell
- Customizable Fuel Cell Stack Components for Diverse Applications
- Customizable CO2 Reduction Flow Cell for NRR ORR and CO2RR Research
- FS Electrochemical Hydrogen Fuel Cells for Diverse Applications
- Customizable PEM Electrolysis Cells for Diverse Research Applications
People Also Ask
- What is the precaution regarding temperature when using an all-PTFE electrolytic cell? Essential Thermal Safety Tips
- What functions do electrolytic cells perform in PEC water splitting? Optimize Your Photoelectrochemical Research
- What is the purpose of the double-layer structure in the H-type electrolytic cell? Achieve Precise Thermal Control
- What are the primary functions of a high-performance electrolytic cell in the eCO2R process? Optimize Your Lab Results
- What is the purpose of using a frit glass tube in a three-electrode cell? Enhance Vanadium Redox Testing Accuracy



















