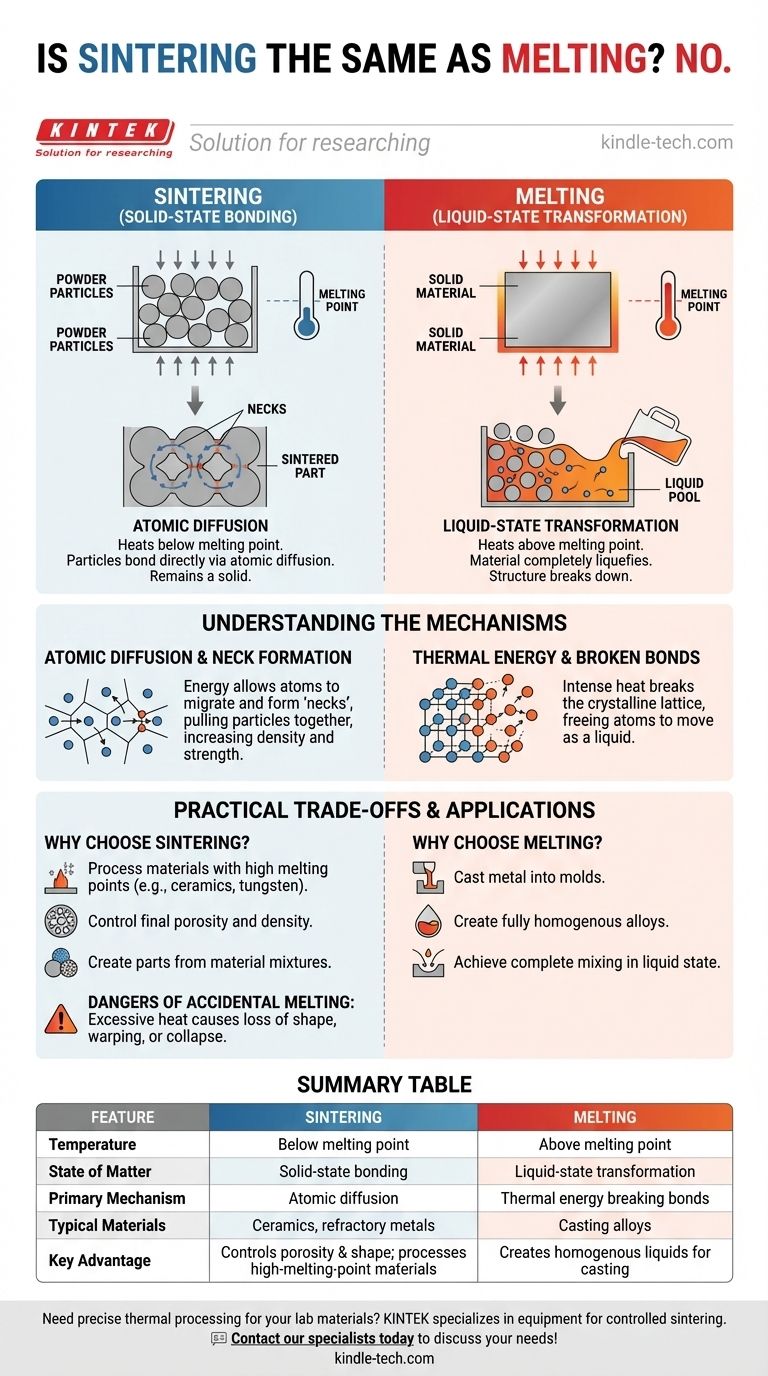
No, sintering is fundamentally different from melting. While both processes use high temperatures to consolidate materials, they operate on opposite sides of a critical threshold. Sintering carefully heats a powdered material to below its melting point, causing the individual particles to fuse together while remaining in a solid state. Melting, in contrast, heats a material above its melting point until it undergoes a complete phase change into a liquid.
The core distinction lies in the state of matter: Sintering is a solid-state bonding process driven by atomic diffusion, whereas melting is a liquid-state transformation that completely breaks down the material's solid structure.

The Core Distinction: State of Matter
To understand the difference, we must first look at how each process treats the physical state of the material.
Sintering: A Solid-State Process
Sintering begins with a compacted mass of powder, often referred to as a "green body."
Heat is applied, but the temperature is precisely controlled to stay below the material's melting point.
The material never becomes a liquid. Instead, the particles bond directly with one another, gradually reducing the empty space (porosity) between them and increasing the overall density and strength of the part.
Melting: A Liquid-State Process
Melting involves heating a material until it reaches or exceeds its melting temperature.
This high level of thermal energy overcomes the atomic bonds holding the solid in a fixed structure, causing a complete phase transition into a liquid.
The end result of melting is not a shaped part, but a homogenous, formless liquid that can then be used in other processes, such as casting.
Understanding the Underlying Mechanisms
The "why" behind this difference comes down to what is happening at an atomic level.
How Sintering Fuses Particles
The driving force behind sintering is atomic diffusion. As the powdered material heats up, its atoms become more energetic.
This energy allows atoms to migrate across the boundaries where individual particles touch, forming small "necks" or bridges between them.
Over time, these necks grow, pulling the particles closer together and creating a strong, solid mass from the loose powder. Pressure is sometimes applied to accelerate this process.
How Melting Creates a Liquid
Melting relies solely on thermal energy to achieve its goal.
The heat becomes so intense that it completely breaks the crystalline lattice that gives the solid its form.
Once these bonds are broken, the atoms are free to move, resulting in the material taking on the fluid properties of a liquid.
Understanding the Practical Trade-offs
Choosing between these processes depends entirely on the material and the desired outcome. The consequences of confusing them can be significant.
Why Choose Sintering?
Sintering is the go-to method for processing materials with extremely high melting points, like tungsten or ceramics, where melting would be impractical or prohibitively expensive.
It also provides unique control over the final part's properties, such as creating components with a specific, engineered level of porosity.
Finally, it allows for the creation of parts from mixtures of materials that would not properly alloy in a liquid state.
The Dangers of Accidental Melting
The most common failure in sintering is applying excessive heat. If the temperature exceeds the melting point, even slightly, the material begins to liquefy.
This can cause the part to lose its shape, warp, or collapse.
Even partial, localized melting can compromise the part's internal structure and mechanical integrity. For this reason, precise temperature control is absolutely critical in any sintering operation.
Making the Right Choice for Your Application
Selecting the correct thermal process is essential for achieving your desired outcome in manufacturing or materials science.
- If your primary focus is creating parts from high-temperature powders (like ceramics or refractory metals): Sintering is the correct process, as it avoids the massive energy costs and challenges of full liquefaction.
- If your primary focus is casting metal into a mold or creating a fully homogenous alloy: Melting is required to achieve the necessary liquid state for pouring and complete mixing.
- If your primary focus is controlling the final density and porosity of a component: Sintering offers precise control over these properties in a way that melting and casting cannot.
Ultimately, understanding this distinction between solid-state fusion and liquid-state transformation is the key to mastering thermal processing.
Summary Table:
| Feature | Sintering | Melting |
|---|---|---|
| Temperature | Below melting point | Above melting point |
| State of Matter | Solid-state bonding | Liquid-state transformation |
| Primary Mechanism | Atomic diffusion | Thermal energy breaking bonds |
| Typical Materials | Ceramics, refractory metals | Casting alloys |
| Key Advantage | Controls porosity & shape; processes high-melting-point materials | Creates homogenous liquids for casting |
Need precise thermal processing for your lab materials?
Understanding the critical difference between sintering and melting is essential for successful outcomes with ceramics, metals, and powdered materials. KINTEK specializes in the lab equipment and consumables needed for controlled sintering processes and high-temperature applications.
Let our experts help you select the right solution to achieve the desired density, strength, and properties in your components.
Contact our thermal processing specialists today to discuss your specific laboratory needs!
Visual Guide
Related Products
- Dental Porcelain Zirconia Sintering Ceramic Furnace Chairside with Transformer
- Spark Plasma Sintering Furnace SPS Furnace
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Vacuum Heat Treat and Sintering Furnace with 9MPa Air Pressure
People Also Ask
- What is the sintering time for zirconia? A Guide to Precise Firing for Optimal Results
- What is the sintering temperature of zirconium? A Guide to the 1400°C-1600°C Range for Dental Labs
- What are the white spots on zirconia after sintering? A Guide to Diagnosing and Preventing Defects
- What is the effect of zirconia sintering temperature? Master the Key to Strength and Stability
- Can you change the color of zirconia crowns? Understanding the Permanent Nature of Zirconia



















