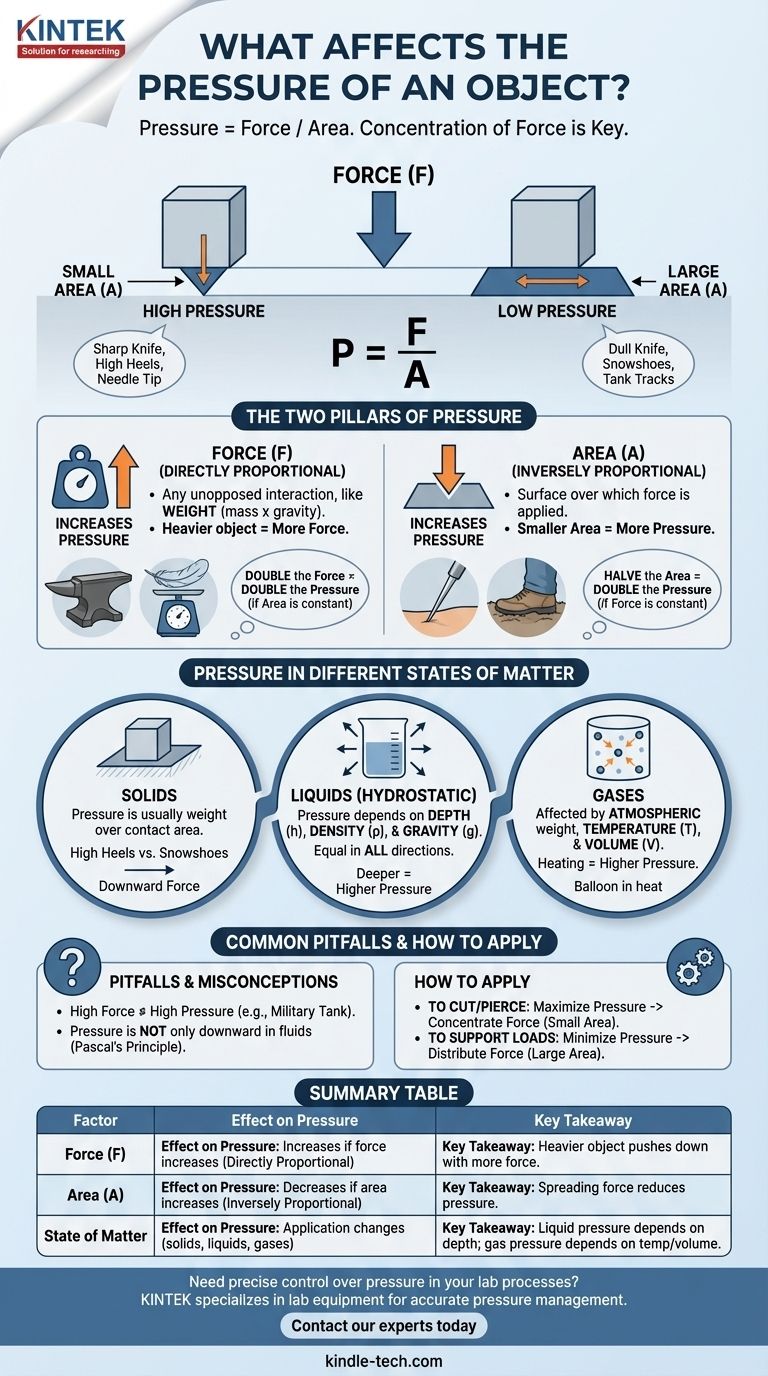
In short, pressure is determined by two primary factors: the amount of force applied and the area over which that force is distributed. Pressure is simply the measure of how concentrated a force is. A massive force spread over a huge area can result in very low pressure, while a tiny force focused on a pinpoint area can create immense pressure.
The central takeaway is that pressure is not about the total force, but its concentration. To increase pressure, you must either increase the force or, more effectively, decrease the area of application. Conversely, to decrease pressure, you spread the same force over a larger area.

The Two Pillars of Pressure: Force and Area
To truly grasp how pressure works, we must analyze its two core components. The relationship between them is defined by the simple formula: Pressure = Force / Area.
Understanding Force
Force is any interaction that, when unopposed, will change the motion of an object. In many common scenarios involving pressure, this force is simply the weight of an object.
Weight is the force generated by gravity acting on an object's mass. A heavier object exerts more force than a lighter one.
The Critical Role of Area
Area is the most intuitive and often most important factor in managing pressure. It refers to the specific surface area over which the force is applied.
This is why a sharp knife cuts easily while a dull one does not. Both may be pushed with the same force, but the sharp knife concentrates that force onto a microscopic edge, creating incredibly high pressure that can sever material. The dull knife spreads the force over a wider area, resulting in low pressure.
The Mathematical Relationship
The formula P = F/A clearly shows that pressure (P) is directly proportional to force (F) and inversely proportional to area (A).
This means if you double the force while keeping the area constant, you double the pressure. However, if you halve the area while keeping the force constant, you also double the pressure.
Pressure in Different States of Matter
While the concept of force over area is universal, its application changes depending on whether you are dealing with solids, liquids, or gases.
Pressure from Solids
For solid objects, pressure is most straightforward. The force is typically the object's weight, and the area is the surface in direct contact with the ground or another object.
A person wearing high heels concentrates their entire body weight onto two tiny points, exerting high pressure on a floor. The same person wearing snowshoes spreads that same weight over a very large area, resulting in low pressure that allows them to walk on top of snow.
Pressure in Liquids (Hydrostatic Pressure)
In a fluid like water, pressure is not just exerted downwards but equally in all directions. The pressure at any point within a liquid is determined by three factors:
- Depth (h): The deeper you go, the more fluid is above you, and the greater the weight of that fluid column. This is why your ears feel pressure at the bottom of a deep pool.
- Density of the liquid (ρ): Denser fluids (like mercury) will exert more pressure at the same depth than less dense fluids (like water).
- Gravitational acceleration (g): The force of gravity pulls the fluid down, creating the pressure.
Pressure in Gases
Gases also exert pressure. We are constantly living under atmospheric pressure, which is the pressure caused by the weight of the entire column of air in the atmosphere above us.
For a gas inside a container, its pressure is also affected by its temperature and volume. Heating a gas in a sealed container increases its pressure because the gas molecules move faster and collide with the container walls more forcefully and frequently.
Common Pitfalls and Misconceptions
Understanding the nuances of pressure helps avoid common errors in thinking.
Confusing High Force with High Pressure
A very large force does not automatically mean high pressure. A military tank is incredibly heavy (exerting a massive force), but its weight is distributed across wide tracks.
The resulting pressure on the ground is surprisingly low—often less than that of a human foot—which is why it doesn't sink into soft ground.
Assuming Pressure is Only Downward
While the weight of a solid object creates downward pressure, pressure within a fluid (a liquid or gas) is exerted equally in all directions at any given depth.
This principle, known as Pascal's principle, is the foundation for hydraulic systems like car brakes and lifts.
How to Apply This Knowledge
Your specific goal will determine which factor—force or area—you need to manipulate.
- If your primary focus is to cut, pierce, or puncture: You must maximize pressure by concentrating force onto the smallest possible area (e.g., a sharp needle, a knife edge, or a nail tip).
- If your primary focus is to support a heavy load without sinking: You must minimize pressure by distributing the force over the largest possible area (e.g., building foundations, wide tires on a tractor, or snowshoes).
- If your primary focus is underwater or aviation engineering: You must account for how pressure changes dramatically with depth in liquids or altitude in the atmosphere.
- If your primary focus is designing a sealed system with gases: You must manage the relationship between pressure, volume, and temperature to prevent system failure.
By understanding the fundamental relationship between force and area, you gain the ability to engineer and control physical interactions in any environment.
Summary Table:
| Factor | Effect on Pressure | Key Takeaway |
|---|---|---|
| Force (F) | Increases if force increases (directly proportional) | A heavier object pushes down with more force. |
| Area (A) | Decreases if area increases (inversely proportional) | Spreading force over a larger area reduces pressure. |
| State of Matter | Application changes (solids, liquids, gases) | Liquid pressure depends on depth; gas pressure depends on temperature/volume. |
Need precise control over pressure in your lab processes?
Understanding the relationship between force and area is critical for applications like material testing, filtration, and reactor design. KINTEK specializes in providing high-quality lab equipment and consumables that help you accurately manage pressure for reliable, repeatable results.
Contact our experts today to discuss how our solutions can enhance your laboratory's capabilities and efficiency.
Visual Guide
Related Products
- Automatic Lab Cold Isostatic Press CIP Machine Cold Isostatic Pressing
- Electric Lab Cold Isostatic Press CIP Machine for Cold Isostatic Pressing
- Manual High Temperature Heated Hydraulic Press Machine with Heated Plates for Lab
- Automatic High Temperature Heated Hydraulic Press Machine with Heated Plates for Lab
- 24T 30T 60T Heated Hydraulic Press Machine with Heated Plates for Laboratory Hot Press
People Also Ask
- What are the different types of sintering process? Choose the Right Method for Your Material
- What is the product of plasma pyrolysis? A Clean Syngas and Inert Slag from Waste
- What is sintering process related to? Mastering Powder-to-Solid Manufacturing
- What is the best catalyst for pyrolysis? A Strategic Guide to Maximizing Bio-Oil Quality and Yield
- Is hot isostatic pressing the same as sintering? Unlock Superior Density and Performance
- What is sintering in semiconductor? Creating Reliable Ohmic Contacts for High-Performance Chips
- What are the advantages of liquid phase sintering? Achieve Faster, Denser, and Stronger Parts
- What is the sizing process in sintering? Master Dimensional Control for Precision Parts



















