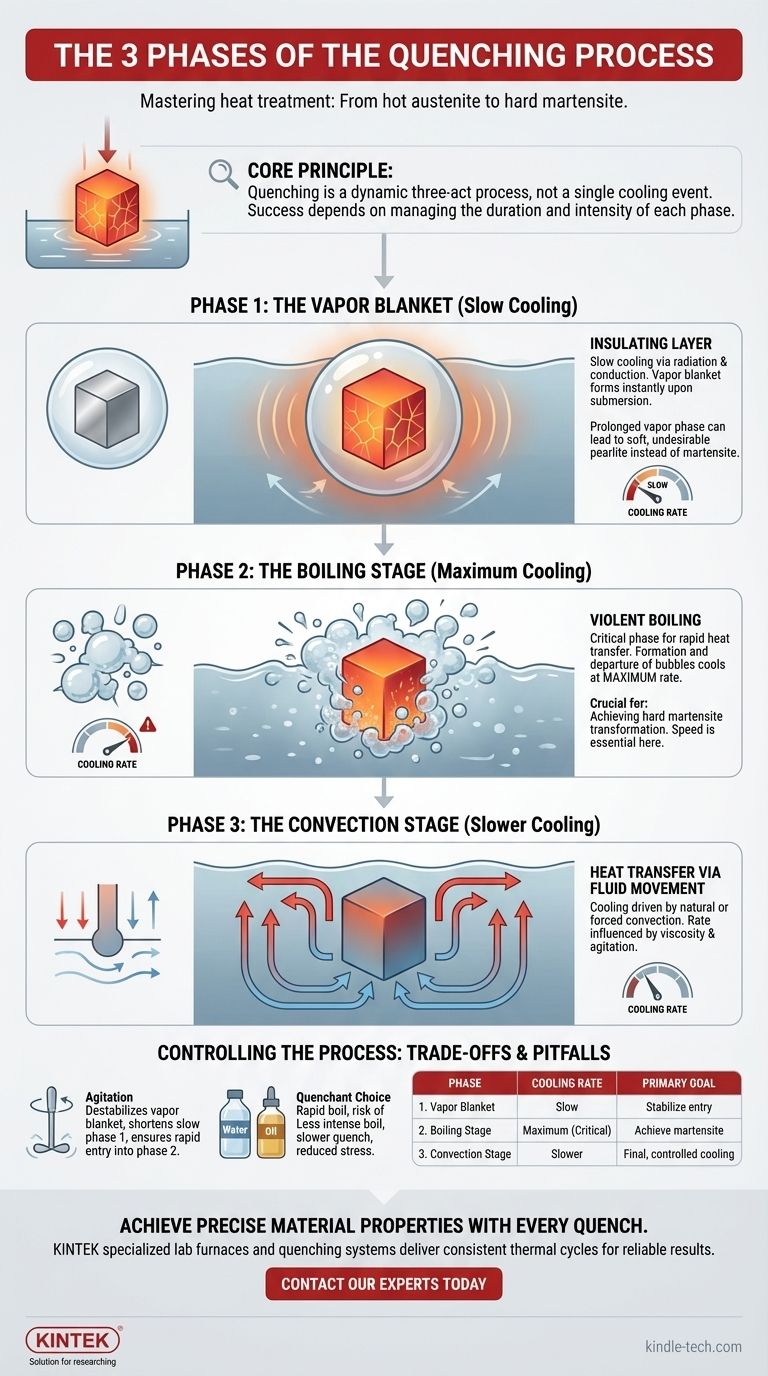
The three distinct phases of quenching are the vapor phase, the boiling phase, and the convection phase. As a hot metal part is submerged in a liquid quenchant, it does not cool at a steady rate. Instead, it passes through these three stages, each with a dramatically different speed of heat transfer, which ultimately determines the final properties of the material.
The core principle to understand is that quenching is not a single cooling event, but a dynamic three-act process. The success or failure of the heat treatment hinges on managing the duration and intensity of each phase, particularly the violent boiling phase where the fastest cooling occurs.

The Purpose of Quenching: Freezing a Moment in Time
To understand the quenching phases, you must first understand the goal. In heat treating, a metal like steel is heated to a high temperature, causing its internal crystal structure to change into a form called austenite.
The goal of quenching is to cool the metal so rapidly that this new structure is "frozen" in place, transforming into a very hard, strong, and brittle structure known as martensite. The speed of this cooling is everything.
A Phase-by-Phase Breakdown of Quenching
The cooling curve during quenching is not linear. It is defined by three distinct physical phenomena that occur at the surface of the part.
Phase 1: The Vapor Blanket (Slow Cooling)
When the hot part first enters the liquid, it's so hot that it instantly vaporizes the quenchant it touches. This creates a thin, stable film of vapor that completely surrounds the part.
This vapor blanket acts as an insulating layer, dramatically slowing down heat transfer. Cooling in this phase is relatively slow and occurs mainly through radiation and conduction across the vapor.
Phase 2: The Boiling Stage (Maximum Cooling)
As the part's surface cools slightly, the vapor blanket becomes unstable and collapses. The liquid quenchant now makes direct contact with the hot metal, causing it to boil violently.
This is the most critical stage of the process. The formation and departure of countless bubbles at the surface transfers heat away from the part at the maximum possible rate. It is the speed of this phase that primarily determines whether hard martensite will form.
Phase 3: The Convection Stage (Slower Cooling)
Once the part's surface temperature drops below the boiling point of the quenchant, boiling stops.
From this point on, cooling is driven by convection. The warmer liquid in contact with the part rises, and cooler liquid moves in to take its place, carrying heat away. The cooling rate drops off significantly compared to the boiling phase and is influenced by the quenchant's viscosity and the degree of agitation.
Understanding the Trade-offs and Pitfalls
Controlling the transition between these phases is the key to successful heat treating. Failing to do so can lead to undesirable outcomes.
The Danger of a Prolonged Vapor Phase
If the insulating vapor blanket (Phase 1) persists for too long, the cooling rate can drop below the critical cooling rate required for the steel.
Instead of forming hard martensite, the slow cooling allows softer, less desirable structures (like pearlite) to form. This results in a part that is not as hard as intended.
The Impact of Quenchant Choice
Different liquids have different boiling points and heat transfer capabilities, which directly impacts the three phases.
Water creates a very intense and rapid boiling phase but can also cause a more stable vapor phase. Oil has a less intense boiling phase, providing a slower quench that reduces the risk of cracking or distortion in sensitive parts.
The Role of Agitation
Agitating the quenchant (stirring it or moving the part within it) is a critical technique. It helps to destabilize the vapor blanket, shortening the slow first phase and ensuring the part enters the rapid boiling phase more quickly and uniformly.
Making the Right Choice for Your Goal
By understanding these phases, you can diagnose problems and control the process to achieve a specific outcome.
- If your primary focus is maximum hardness: You must ensure the cooling rate during the boiling phase is fast enough to exceed the steel's critical threshold, which often requires minimizing the duration of the initial vapor phase through agitation.
- If your primary focus is preventing distortion or cracks: You may need a slower quenchant, like oil, which creates a less violent boiling phase and reduces thermal stress on the part.
- If you are troubleshooting a soft part: The most likely cause is a prolonged vapor phase or an insufficiently rapid boiling phase, often solved by increasing agitation or checking the temperature and condition of your quenchant.
By understanding these three distinct cooling phases, you move from simply quenching a part to truly engineering its final properties.
Summary Table:
| Phase | Key Event | Cooling Rate | Primary Goal |
|---|---|---|---|
| 1. Vapor Blanket | Insulating vapor film forms | Slow | Stabilize part entry |
| 2. Boiling Stage | Violent boiling at surface | Maximum (Critical) | Achieve martensite transformation |
| 3. Convection Stage | Heat transfer via fluid movement | Slower | Final, controlled cooling |
Achieve precise material properties with every quench. The right lab equipment is critical for controlling the vapor, boiling, and convection phases. KINTEK specializes in lab furnaces and quenching systems that deliver consistent thermal cycles for reliable results.
Contact our experts today to discuss how our solutions can enhance your heat treatment process and ensure your materials meet exact specifications.
Visual Guide
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Brazing Furnace
- Vacuum Heat Treat Furnace and Levitation Induction Melting Furnace
- Molybdenum Vacuum Heat Treat Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
People Also Ask
- Why is a vacuum drying oven essential in the fabrication of Li8/7Ti2/7V4/7O2 electrodes? Prevent Battery Degradation
- Why can't convection and conduction occur in vacuum? The Critical Role of Matter in Heat Transfer
- What are the applications of vacuum furnace? Achieve Purity and Precision in High-Temperature Processing
- What are the different types of annealing in semiconductors? A Guide to Choosing the Right Thermal Process
- What is the process of vacuum melting? Achieve Ultra-Pure Metals for Critical Applications
- What is the primary role of a high-temperature industrial furnace in the carbothermal reduction process?
- What is the main purpose of a furnace? A Guide to Heating, Comfort, and Material Transformation
- What are the defects of sintering? Avoid Costly Flaws in Your Powdered Metal Parts



















