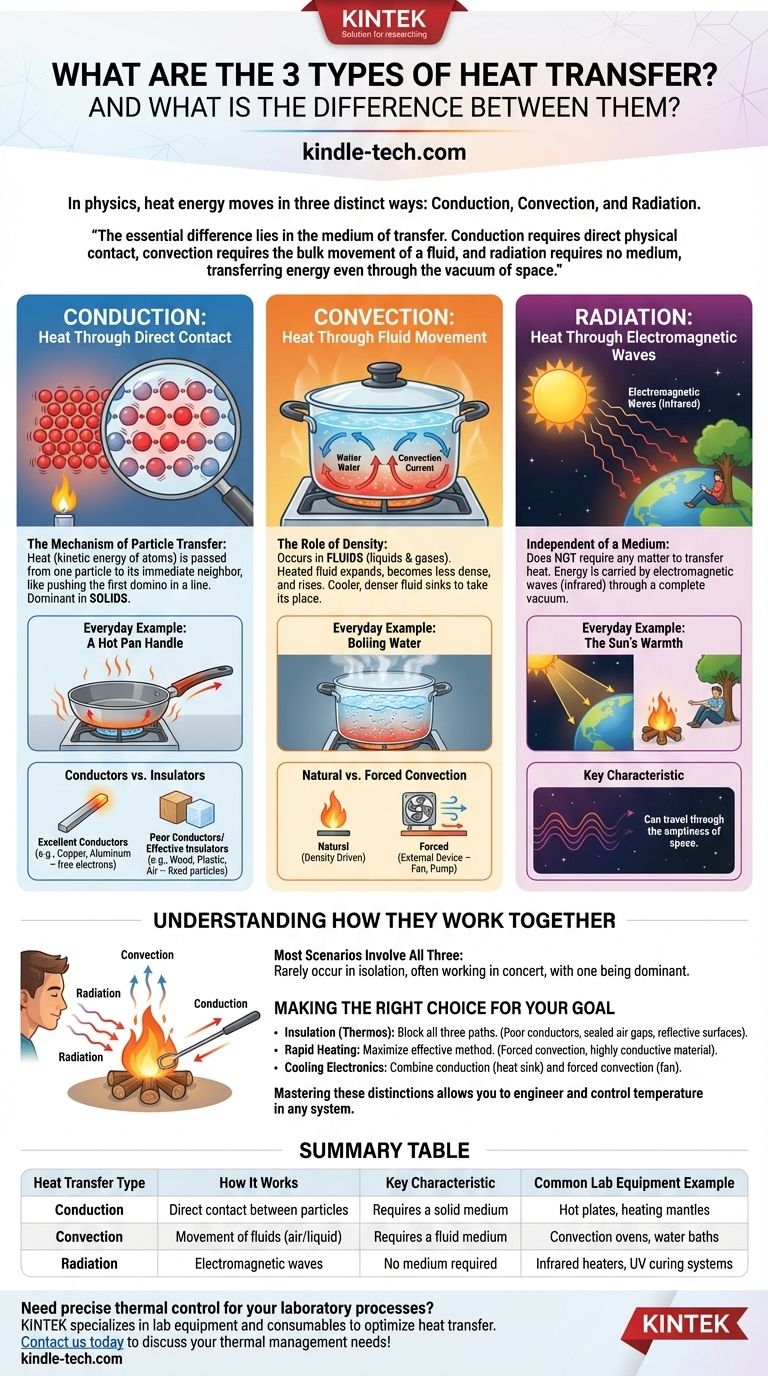
In physics, heat energy moves in three distinct ways: conduction, convection, and radiation. Conduction is heat transfer through direct contact, convection is heat transfer through the movement of fluids (like air or water), and radiation is heat transfer through electromagnetic waves, which requires no medium at all.
The essential difference lies in the medium of transfer. Conduction requires direct physical contact, convection requires the bulk movement of a fluid, and radiation requires no medium, transferring energy even through the vacuum of space.

Conduction: Heat Through Direct Contact
The Mechanism of Particle Transfer
Conduction occurs when heat, which is the kinetic energy of atoms, is passed from one particle to its immediate neighbor. Imagine a line of tightly packed dominos; when you push the first one, the energy is transferred all the way down the line without any single domino traveling the full distance.
This is why conduction is the dominant form of heat transfer in solids, where particles are held in a fixed lattice and can only vibrate in place.
Everyday Example: A Hot Pan Handle
When you place a metal pan on a hot stove, the burner transfers heat to the bottom of the pan via conduction. The atoms in the pan begin to vibrate rapidly, passing that energy along from one atom to the next until the entire pan, including the handle, becomes hot.
Conductors vs. Insulators
Materials differ greatly in their ability to conduct heat. Metals like copper and aluminum are excellent conductors because their electrons are free to move and transfer energy quickly.
Materials like wood, plastic, and air are poor conductors, which makes them effective insulators. This is why pan handles are often made of plastic and why winter coats use trapped air to keep you warm.
Convection: Heat Through Fluid Movement
The Role of Density
Convection only happens in fluids (liquids and gases). When a portion of a fluid is heated, it expands, becomes less dense, and rises. Cooler, denser fluid from above sinks to take its place, gets heated, and also rises.
This continuous circulation, known as a convection current, is a highly effective way to transfer heat throughout a fluid.
Everyday Example: Boiling Water
When you boil water in a pot, the water at the bottom is heated by conduction from the pot. This hot water rises, and the cooler, denser water from the top sinks to the bottom to be heated. You can see these convection currents as the water begins to churn and roll before it boils.
Natural vs. Forced Convection
The boiling water example is natural convection, driven purely by density differences.
Forced convection occurs when an external device, like a fan or pump, moves the fluid to accelerate heat transfer. A convection oven uses a fan to circulate hot air for even cooking, and a computer's CPU cooler uses a fan to blow air across a hot metal sink.
Radiation: Heat Through Electromagnetic Waves
Independent of a Medium
Radiation is unique because it does not require any matter to transfer heat. Energy is carried by electromagnetic waves (specifically, infrared radiation), which can travel through a complete vacuum.
This is the only way heat can travel through the emptiness of space.
Everyday Example: The Sun's Warmth
The most powerful example of radiation is the sun. Its energy travels 93 million miles through the vacuum of space to warm the Earth. You also feel heat radiation when you stand near a campfire or a hot stove burner, even without touching it or being directly in the path of rising hot air.
Understanding How They Work Together
Most Scenarios Involve All Three
In the real world, these three modes of heat transfer rarely occur in complete isolation. They almost always work in concert, though one is often dominant.
Consider a campfire. You feel the radiation on your face. The air directly above the flames is heated and rises due to convection. If you stick a metal poker into the fire, its handle will eventually become hot due to conduction.
One Mode Is Often Dominant
Solving a thermal problem involves identifying the primary mode of transfer. To heat a solid metal bar, conduction is key. To heat a room with a radiator, convection is the dominant process. To receive energy from the sun, radiation is the only method that works.
Making the Right Choice for Your Goal
Understanding these principles allows you to control heat transfer for a specific purpose.
- If your primary focus is insulation (keeping something hot or cold): You must block all three paths by using poor conductors (insulators), sealing air gaps (preventing convection), and using reflective surfaces (blocking radiation), which is exactly how a thermos flask works.
- If your primary focus is rapid heating: You must maximize the most effective transfer method, such as using forced convection (a fan oven) or choosing a highly conductive material for direct contact (a copper-bottomed pan).
- If your primary focus is cooling an electronic component: You combine conduction to pull heat away from the chip into a metal heat sink and forced convection to move cool air across the sink's fins.
By mastering the distinction between conduction, convection, and radiation, you gain the ability to engineer and control temperature in any system.
Summary Table:
| Heat Transfer Type | How It Works | Key Characteristic | Common Lab Equipment Example |
|---|---|---|---|
| Conduction | Direct contact between particles | Requires a solid medium | Hot plates, heating mantles |
| Convection | Movement of fluids (air/liquid) | Requires a fluid medium | Convection ovens, water baths |
| Radiation | Electromagnetic waves | No medium required | Infrared heaters, UV curing systems |
Need precise thermal control for your laboratory processes? KINTEK specializes in lab equipment and consumables designed to optimize heat transfer for your specific applications. Whether you require conduction-based heating, convection ovens for uniform temperature distribution, or radiation systems for clean energy transfer, our experts can help you select the right solution. Contact us today to discuss how we can enhance your lab's thermal management and efficiency!
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1800℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What are the applications of DC sputtering? Achieve High-Quality Conductive Coatings for Your Industry
- What safety precautions should be taken during the sintering process? Essential Lab Safety Guide
- How do separate internal compartments in ultra-low freezers improve efficiency? Enhance Stability and Reduce Costs
- How is heat transferred in a furnace? Master Radiation, Convection & Conduction
- What is the carbon footprint of diamond mining? Uncovering the True Environmental and Ethical Cost
- What temperature is brazing material? Master the Heat for Perfect Metal Joints
- What are the toxicity and safety issues of carbon nanotubes? Understanding the Physical Risks of Nanomaterials
- What technical issues does a vacuum drying oven solve in AAS mortar experiments? Ensure Purity and Accuracy



















