At its core, electrochemical deposition (ECD) is a method for creating thin films and coatings that offers an exceptional combination of precision, cost-effectiveness, and low-temperature operation. Unlike high-energy, vacuum-based techniques, ECD builds films atom by atom from a liquid solution, giving engineers and scientists a unique level of control over the final product's thickness and structure.
The central advantage of electrochemical deposition is its ability to produce high-quality, conformal coatings on complex surfaces at room temperature and low cost, a capability set that is difficult to achieve with alternative methods like PVD or CVD.
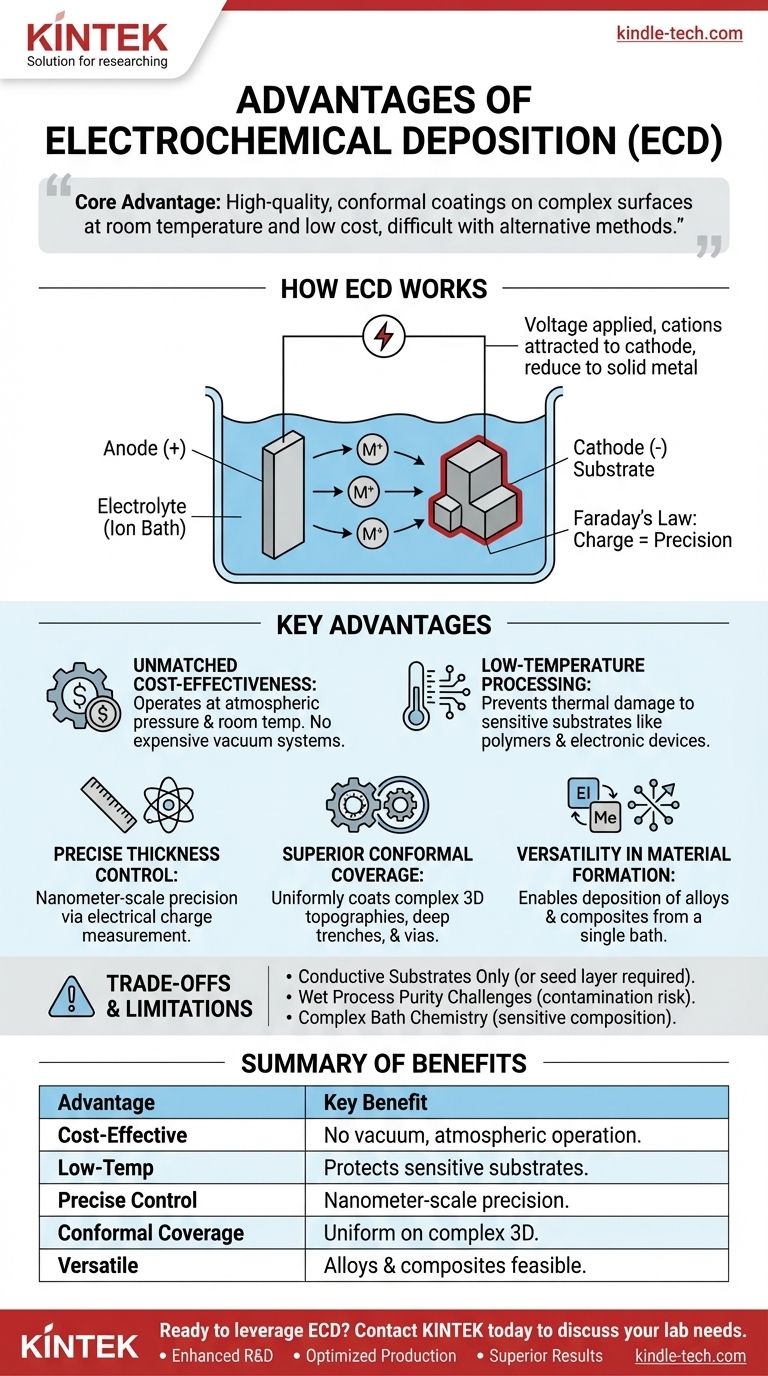
How Electrochemical Deposition Works
To appreciate its advantages, it is first necessary to understand the fundamental mechanism of ECD, which is remarkably straightforward.
The Basic Setup
An electrochemical deposition system consists of a simple electrochemical cell containing a liquid electrolyte. This solution contains dissolved ions of the material you wish to deposit.
Two electrodes are submerged in this bath: the anode (positive electrode) and the cathode (negative electrode), which is the substrate you want to coat.
Deposition Driven by Potential
When a voltage is applied, positive ions (cations) in the electrolyte are attracted to the negative cathode. At the substrate's surface, these ions gain electrons and are "reduced" to their solid metallic state, depositing onto the substrate.
Precision Through Charge
According to Faraday's laws of electrolysis, the amount of material deposited is directly proportional to the total electrical charge passed through the cell. This physical law is the key to ECD's remarkable precision.
Key Advantages of Electrochemical Deposition
The principles of ECD give rise to several powerful advantages, making it the ideal choice for specific applications where other methods fall short.
Unmatched Cost-Effectiveness
ECD systems operate at atmospheric pressure and typically near room temperature. This eliminates the need for expensive high-vacuum chambers, high-power sources, and complex gas handling systems that define methods like physical vapor deposition (PVD) or chemical vapor deposition (CVD).
Low-Temperature Processing
The ability to deposit films at or near room temperature is a critical advantage. It prevents thermal damage to sensitive substrates like polymers, plastics, or fully fabricated electronic devices with pre-existing components.
Precise, Atomic-Level Thickness Control
By simply measuring the total charge (current multiplied by time), one can control film thickness with nanometer-scale precision. This allows for the creation of ultra-thin layers and multilayered structures with a level of control that is difficult to replicate with thermally driven processes.
Superior Conformal Coverage
The electric field lines in the solution naturally wrap around the substrate, driving ion deposition uniformly across complex, three-dimensional surfaces. This allows ECD to perfectly coat high-aspect-ratio features like deep trenches, vias, and porous foams—a task where line-of-sight methods like sputtering often fail.
Versatility in Alloy and Compound Formation
Creating an alloy film is as simple as adding ions of different metals into the same electrolyte bath. By controlling the bath chemistry and applied potential, a wide range of metal alloys, composite materials, and even some compound semiconductors can be deposited with specific stoichiometries.
Understanding the Trade-offs and Limitations
No technique is universally superior. Acknowledging the limitations of ECD is crucial for making an informed decision.
Substrate and Material Constraints
The primary limitation is that the substrate must be electrically conductive (or be made conductive with a thin seed layer). Furthermore, the range of depositable materials is limited to those that can be electrochemically reduced from a stable electrolyte solution, which primarily includes metals, alloys, and some semiconductors. Many ceramics and oxides are not suitable for direct ECD.
The Challenge of Purity
Because ECD is a "wet" chemical process, the electrolyte is a potential source of contamination. Impurities in the chemicals or water can be co-deposited into the film. Achieving the ultra-high purity levels possible in high-vacuum systems requires meticulous bath preparation and maintenance.
Complexity of Bath Chemistry
The quality of the deposited film is highly sensitive to the electrolyte's composition, including pH, temperature, additives, and ion concentration. Developing and maintaining an optimized and stable bath can require significant expertise and process control.
Making the Right Choice for Your Goal
Selecting a deposition technique depends entirely on your project's specific priorities and constraints.
- If your primary focus is cost and scalability: ECD is often the superior choice for large-area coatings or high-volume production due to its low equipment cost and atmospheric operation.
- If you are working with temperature-sensitive substrates: The room-temperature nature of ECD is essential for protecting polymers, organic electronics, or other delicate components from thermal damage.
- If you need to coat complex, 3D topographies: ECD's excellent conformal coverage is ideal for filling deep trenches, vias, or porous structures where line-of-sight methods fail.
- If precise, nanometer-level thickness control is critical: ECD offers atomic-level precision by simply monitoring the electrical charge, making it perfect for creating quantum dots, superlattices, or other nanostructures.
By understanding its unique advantages and limitations, you can leverage electrochemical deposition as a powerful and targeted tool for advanced material fabrication.

Summary Table:
| Advantage | Key Benefit |
|---|---|
| Cost-Effectiveness | Operates at atmospheric pressure, eliminating expensive vacuum systems. |
| Low-Temperature Processing | Protects sensitive substrates like polymers from thermal damage. |
| Precise Thickness Control | Nanometer-scale control via electrical charge measurement. |
| Superior Conformal Coverage | Uniformly coats complex 3D topographies like trenches and vias. |
| Material Versatility | Enables deposition of alloys and composites from a single bath. |
Ready to leverage electrochemical deposition in your lab?
Electrochemical deposition is a powerful tool for creating precise, high-quality coatings. KINTEK specializes in providing the lab equipment and consumables you need to implement ECD and other advanced fabrication techniques successfully.
We help you achieve:
- Enhanced R&D Capabilities: Access the right tools for material science innovation.
- Optimized Production: Scale your processes with reliable, cost-effective equipment.
- Superior Results: Achieve the precise, conformal coatings your projects demand.
Contact KINTEK today to discuss your specific laboratory needs and discover how our solutions can drive your research and production forward.
Visual Guide
Related Products
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Electron Beam Evaporation Coating Oxygen-Free Copper Crucible and Evaporation Boat
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Electrolytic Electrochemical Cell for Coating Evaluation
People Also Ask
- What is plasma activated chemical vapour deposition method? A Low-Temperature Solution for Advanced Coatings
- What are the drawbacks of PECVD? Understanding the Trade-offs of Low-Temperature Deposition
- How does RF power create plasma? Achieve Stable, High-Density Plasma for Your Applications
- What are the uses of PECVD? A Guide to Low-Temperature Thin-Film Deposition
- What are the components of PECVD? A Guide to Low-Temperature Thin Film Deposition Systems



















