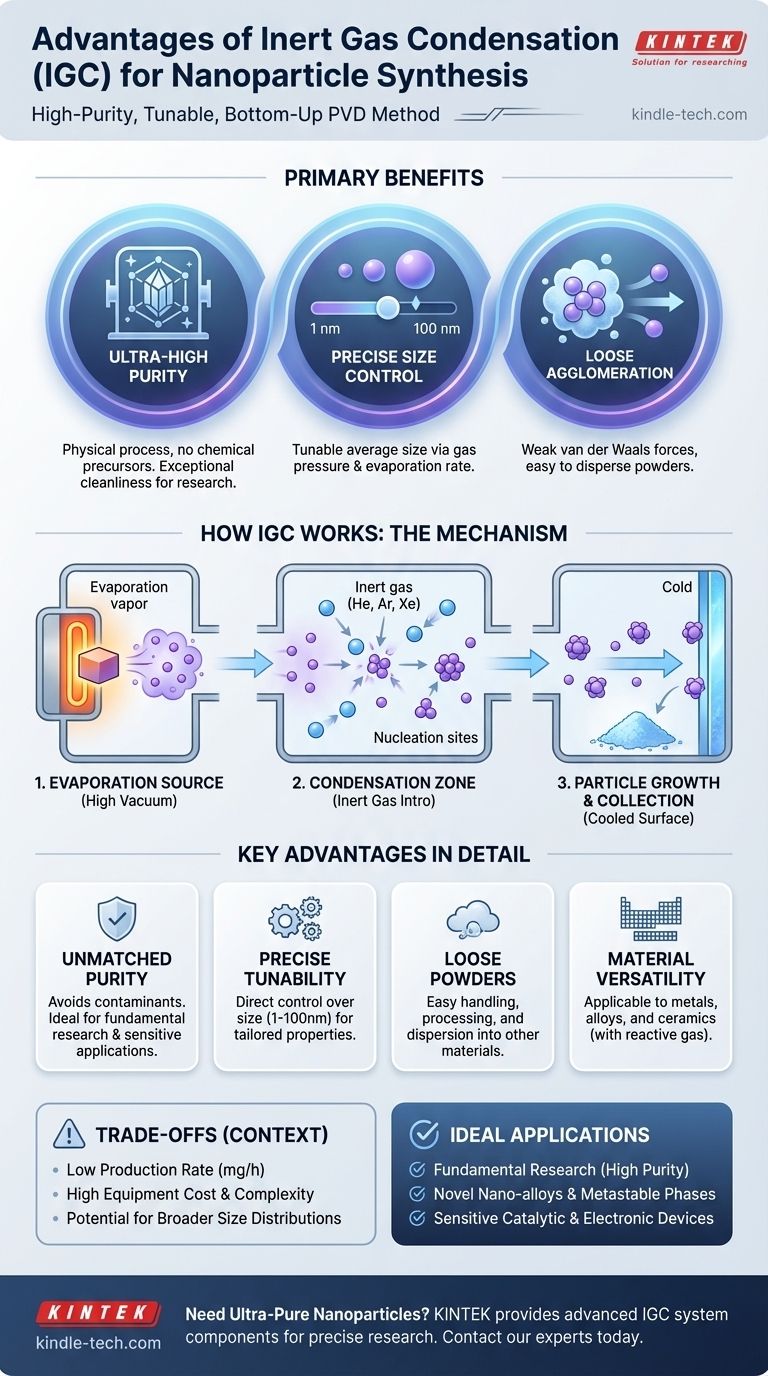
The primary advantages of inert gas condensation (IGC) are its ability to produce exceptionally high-purity nanoparticles with tunable sizes and a low degree of hard agglomeration. This physical vapor deposition (PVD) method achieves this by evaporating a source material in an ultra-clean, high-vacuum environment and then introducing a controlled amount of inert gas, which forces the vapor to condense into nanoscale clusters before they reach a surface.
Inert gas condensation stands out as a "bottom-up" synthesis technique that prioritizes material purity and structural control over production volume. Its strength lies in creating high-quality, loosely-bound nanoparticles ideal for research and specialized applications where chemical contaminants from other methods are unacceptable.

How Inert Gas Condensation Works
To understand the advantages of IGC, it's essential to grasp its fundamental mechanism. The process occurs in a sealed vacuum chamber and consists of two primary stages.
The Evaporation Source
First, a solid source material is heated in a high-vacuum environment until it evaporates, creating a cloud of atoms. This heating can be achieved through various methods, such as thermal resistance heating (like a filament in a lightbulb), electron beam heating, or laser ablation. The key is to generate a stable, controllable flow of atomic vapor.
The Condensation Zone
Next, a low-pressure inert gas (typically Helium, Argon, or Xenon) is introduced into the chamber. The hot atoms from the source material collide with the cool inert gas atoms. These collisions rapidly remove kinetic energy from the evaporated atoms, causing them to become supersaturated and nucleate into tiny clusters or nanoparticles in the gas phase.
Particle Growth and Collection
These newly formed nanoparticles are then carried by the gentle flow of the inert gas toward a collection surface, which is often cryogenically cooled. During this transit, particles can grow slightly through coalescence. Because they form in the gas and are collected gently, they tend to form very loose, "fluffy" agglomerates that are easily dispersed later.
Key Advantages of the IGC Method
The unique mechanism of IGC gives rise to several distinct advantages over chemical synthesis or mechanical attrition methods.
Unmatched Purity
Because IGC is a purely physical process, it avoids the use of chemical precursors, solvents, or surfactants. The entire synthesis occurs within a high-vacuum chamber, minimizing contamination from the atmosphere. The resulting nanoparticles are composed solely of the evaporated source material, making this the method of choice for creating ultra-pure materials.
Precise Control Over Particle Size
The final average particle size is directly influenced by a few key parameters that can be precisely controlled.
- Inert Gas Pressure: This is the most critical factor. Higher gas pressure leads to more frequent collisions, which cools the atoms faster and results in a higher nucleation rate, producing smaller nanoparticles.
- Evaporation Rate: A higher evaporation rate increases the density of the atomic vapor, leading to the formation of larger nanoparticles.
This tunability allows researchers to systematically produce particles in a desired size range, typically between 1 and 100 nanometers.
Loosely Agglomerated Powders
Unlike many wet-chemical methods where particles crash out of a solution and form hard, tightly bound agglomerates, IGC produces nanoparticles that are loosely held together by weak van der Waals forces. This makes the resulting nanopowder much easier to handle, process, and disperse into other materials or solutions for subsequent applications.
Versatility in Material Synthesis
The IGC method is extremely versatile and can be applied to any material that can be evaporated. This includes a vast range of pure metals, metal alloys, and intermetallic compounds. By introducing a small amount of reactive gas (like oxygen or nitrogen) along with the inert gas, it's also possible to synthesize ceramic nanoparticles like oxides and nitrides.
Understanding the Trade-offs
No method is perfect, and IGC's primary advantages come with significant trade-offs that limit its use cases.
Low Production Rate
The most significant disadvantage of inert gas condensation is its very low yield. Production rates are typically on the order of milligrams to a few grams per hour. This makes the process impractical and cost-prohibitive for any application requiring bulk quantities of nanomaterials.
Equipment Complexity and Cost
IGC requires sophisticated high-vacuum equipment, including vacuum chambers, pumps, power supplies, and potentially cryogenic systems. This machinery is expensive to purchase, operate, and maintain, placing it outside the budget of many labs and making it unsuitable for low-cost industrial production.
Potential for Broader Size Distributions
While the average particle size is controllable, achieving a perfectly uniform, monodisperse sample can be challenging. The random nature of nucleation and coalescence in the gas phase often results in a log-normal size distribution, which may be broader than what can be achieved with certain highly controlled chemical synthesis techniques.
Making the Right Choice for Your Goal
Ultimately, the decision to use IGC depends entirely on your project's primary objective.
- If your primary focus is high-purity materials for fundamental research: IGC is an ideal choice, as it eliminates chemical variables and produces an exceptionally clean product for reliable experiments.
- If your primary focus is creating novel nano-alloys or metastable phases: The rapid quenching inherent to the IGC process allows for the formation of unique nanostructures that cannot be made through conventional metallurgy.
- If your primary focus is bulk industrial production for products like composites or coatings: IGC is unsuitable due to its low yield and high cost; chemical methods like sol-gel, precipitation, or flame spray pyrolysis are far more scalable.
- If your primary focus is developing materials for sensitive catalytic or electronic applications: The high purity and tunable size offered by IGC make it a strong candidate where material quality directly impacts performance.
By understanding these core principles, you can leverage the precision of inert gas condensation for creating advanced materials where quality and purity are paramount.
Summary Table:
| Advantage | Key Benefit |
|---|---|
| Unmatched Purity | Purely physical process avoids chemical contaminants. |
| Precise Size Control | Tunable particle size (1-100 nm) via gas pressure & evaporation rate. |
| Low Agglomeration | Produces loosely-bound, easily-dispersed nanopowders. |
| Material Versatility | Suitable for metals, alloys, and ceramics (with reactive gas). |
Need to synthesize ultra-pure nanoparticles for your research?
KINTEK specializes in providing the advanced lab equipment, including components for inert gas condensation systems, to help you achieve precise material control and high-purity results. Our expertise supports researchers in developing novel nanomaterials for catalytic, electronic, and other sensitive applications where quality is paramount.
Contact our experts today to discuss how we can support your specific nanoparticle synthesis needs.
Visual Guide
Related Products
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- Laboratory Test Sieves and Sieving Machines
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
People Also Ask
- What machine is used to make lab-grown diamonds? Discover the HPHT & CVD Technologies
- How does PACVD equipment improve DLC coatings? Unlock Low Friction and High Heat Resistance
- How are reactants introduced into the reaction chamber during a CVD process? Mastering Precursor Delivery Systems
- How does a Hot Filament Chemical Vapor Deposition (HFCVD) reactor function? Expert Guide to Diamond Film Fabrication
- What is microwave plasma CVD? A Guide to High-Purity Diamond and Material Synthesis