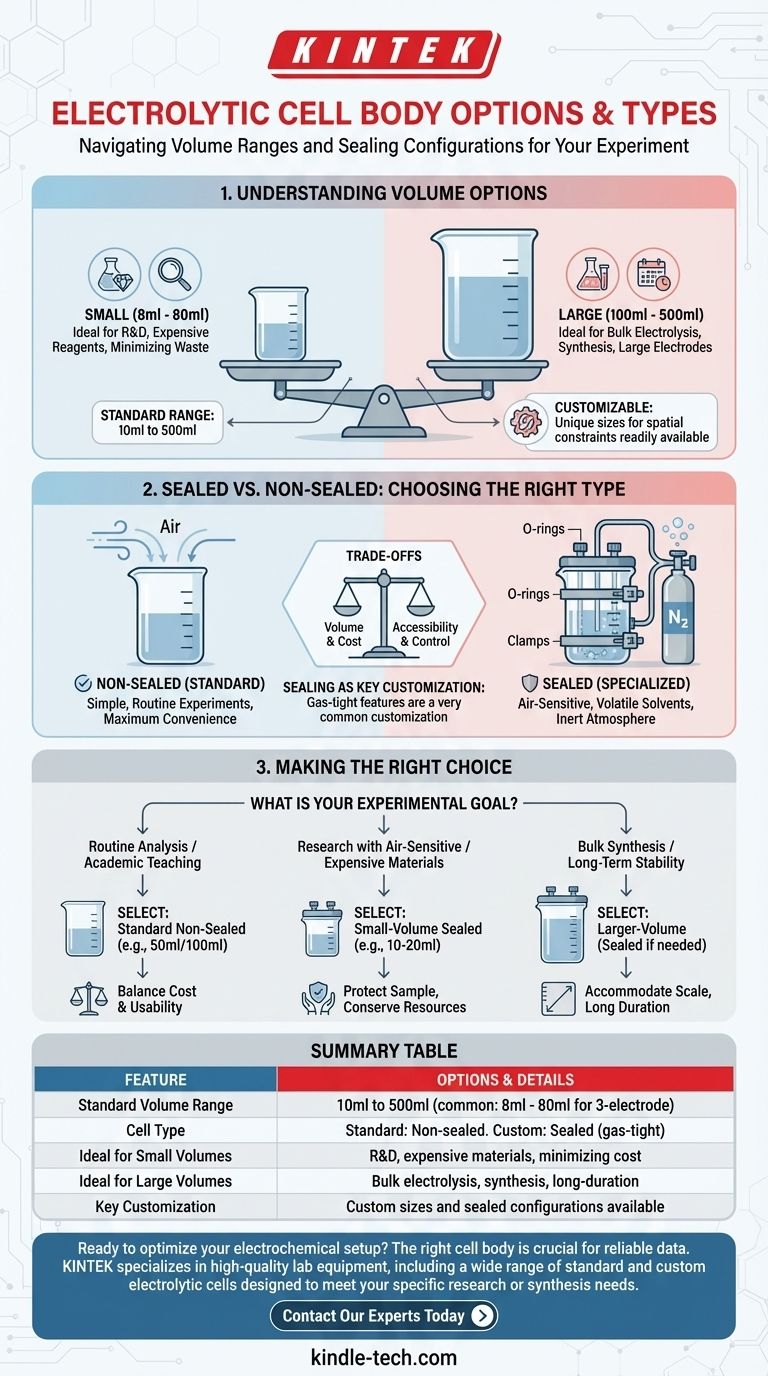
To answer your question directly, electrolytic cell bodies are available in a wide range of volumes, typically from as small as 8ml up to 500ml. The standard configuration is a non-sealed design, but sealed versions can almost always be customized to meet specific experimental needs.
The choice of an electrolytic cell is not merely about its size; it is a critical decision that balances the scale of your experiment, the sensitivity of your reactants, and your operational requirements.

Understanding Cell Volume Options
The volume of your electrolytic cell directly impacts the scale and cost of your experiment. Selecting the right size is the first step in setting up a successful electrochemical system.
Standard Volume Ranges
Standard, off-the-shelf cell bodies typically cover a broad spectrum. You will commonly find options ranging from 10ml to 500ml, with a popular subset for three-electrode systems often falling between 8ml and 80ml.
The Role of Small Volumes
Smaller volumes (e.g., 8-50ml) are ideal for research and development. They are particularly useful when working with expensive electrolytes, catalysts, or novel materials, as they minimize waste and reduce overall experimental cost.
When to Use Large Volumes
Larger volumes (e.g., 100-500ml) are necessary for applications like bulk electrolysis, where the goal is to synthesize a significant quantity of a product. They are also required for long-duration experiments or when using large-format working electrodes.
The Availability of Customization
A key feature offered by most suppliers is the ability to fabricate custom sizes. If your experimental setup has unique spatial constraints or requires a non-standard volume, a custom solution is a readily available option.
Sealed vs. Non-Sealed: Choosing the Right Type
Beyond volume, the most critical distinction is whether the cell is open to the atmosphere or sealed. This choice is dictated entirely by the chemical nature of your system.
The Standard: Non-Sealed Cells
The default and most common configuration is the non-sealed (or open) cell. This design is simpler, easier to assemble, and perfectly suitable for a vast number of experiments where exposure to air is not a concern.
The Specialized: Sealed Cells
A sealed cell is essential for any experiment that is sensitive to the atmosphere. This is critical when you need to prevent oxygen or moisture from interfering with your reaction, work with volatile solvents, or maintain an inert atmosphere by purging with gases like nitrogen or argon.
Sealing as a Key Customization
While non-sealed is the standard, obtaining a sealed version is a very common customization. These designs incorporate features like O-rings, threaded caps, or ground glass joints to ensure a gas-tight environment.
Understanding the Trade-offs
Choosing the right cell body involves balancing practical considerations against experimental requirements.
Volume vs. Material Cost
A larger cell requires more electrolyte and reactant, increasing costs. Always select the smallest volume that comfortably accommodates your electrodes and meets your experimental goals.
Sealed Design vs. Accessibility
Sealed cells provide environmental control but are more complex to set up, clean, and access. Non-sealed cells offer maximum convenience and speed for routine measurements.
Standard vs. Custom Lead Time
Standard, off-the-shelf cells are readily available. Opting for a custom volume or a sealed configuration will almost always involve a longer lead time and potentially higher cost.
Making the Right Choice for Your Experiment
Your specific goal should guide your selection of volume and type.
- If your primary focus is routine analysis or academic teaching: A standard, non-sealed cell in a common size (e.g., 50ml or 100ml) offers the best balance of cost and usability.
- If your primary focus is research with air-sensitive or expensive materials: A small-volume (e.g., 10-20ml) sealed cell is the appropriate choice to protect your sample and conserve resources.
- If your primary focus is bulk synthesis or long-term stability testing: A larger-volume cell, potentially sealed depending on the chemistry, will be necessary to accommodate the scale and duration.
Selecting the correct cell body is the foundational step for acquiring reliable and repeatable electrochemical data.
Summary Table:
| Feature | Options & Details |
|---|---|
| Standard Volume Range | 10ml to 500ml (common: 8ml - 80ml for 3-electrode systems) |
| Cell Type | Standard: Non-sealed (open to air). Custom: Sealed (gas-tight) |
| Ideal for Small Volumes | R&D, expensive materials, minimizing reagent cost |
| Ideal for Large Volumes | Bulk electrolysis, synthesis, long-duration tests |
| Key Customization | Custom sizes and sealed configurations are readily available |
Ready to optimize your electrochemical setup? The right cell body is crucial for reliable data. KINTEK specializes in high-quality lab equipment, including a wide range of standard and custom electrolytic cells designed to meet your specific research or synthesis needs. Contact our experts today to discuss your requirements and ensure you get the perfect cell for your application!
Visual Guide
Related Products
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell for Coating Evaluation
People Also Ask
- What precautions should be taken regarding temperature control for the electrolytic cell? Ensure Safe & Accurate Electrolysis
- What is the correct procedure for installing the electrodes and ion-exchange membrane in the H-type electrolytic cell?
- How do power supply equipment and chemical reagents function together in electrochemical etching? Precision Insights
- What safety precautions should be taken during an experiment with the electrolytic cell? A Guide to Preventing Shocks, Burns, and Fires
- How should a new electrolysis cell be cleaned before first use? Ensure Accurate, Reproducible Results
- What is the primary function of an electrolytic cell in hydrogen production? Learn How It Drives Safe Gas Generation
- What types of electrodes are used in an in-situ Raman electrolytic cell? Optimize for Optical and Electrochemical Control
- What is the immediate post-use cleaning procedure for an electrolysis cell? Prevent Residue Buildup for Accurate Results



















