At its core, vacuum drying is a method for removing moisture from a substance at a much lower temperature than is possible at normal atmospheric pressure. Its primary benefit is the ability to dry heat-sensitive materials gently and efficiently, preventing the damage, degradation, or hazardous situations that can occur with high-heat conventional drying.
The fundamental advantage of vacuum drying is control. By lowering the pressure in a chamber, you lower the boiling point of water, allowing you to remove moisture without exposing your product to destructive high temperatures.

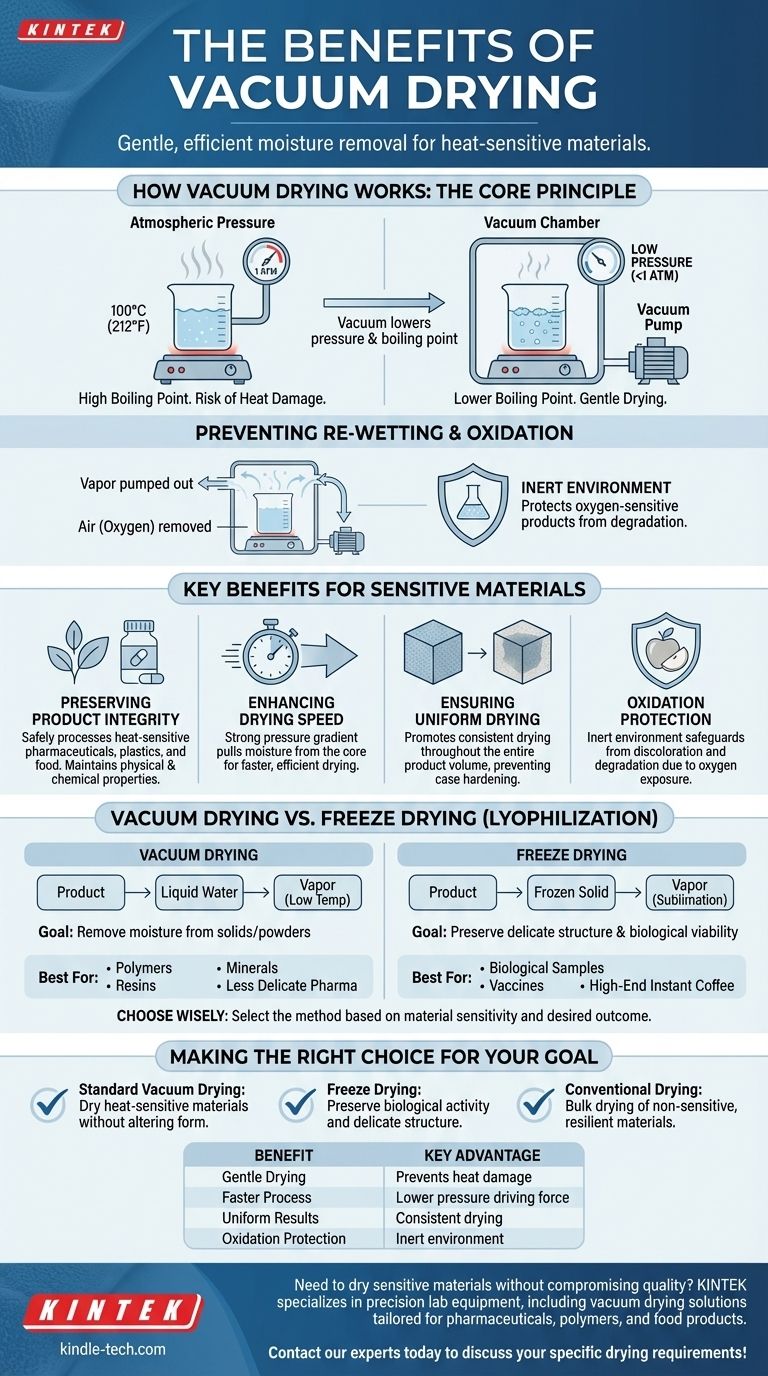
How Vacuum Drying Works: The Core Principle
Vacuum drying is not simply about sucking moisture out. It is a precise process that manipulates physics to achieve a specific outcome.
Lowering the Boiling Point
The boiling point of a liquid is entirely dependent on the pressure surrounding it. At sea level, water boils at 100°C (212°F).
By creating a vacuum, we drastically reduce the ambient pressure. This lower pressure allows water to reach its boiling point—and turn into a vapor—at a much lower temperature, sometimes even at room temperature.
Preventing Re-Wetting and Oxidation
Once the moisture turns into vapor, the vacuum system continuously pumps it out of the chamber. This is critical, as it prevents the vapor from cooling, condensing, and re-wetting the product.
A significant secondary benefit is the removal of air, particularly oxygen. This inert environment protects oxygen-sensitive products from oxidation, which can cause discoloration or degradation.
Key Benefits for Sensitive Materials
The ability to dry materials at low temperatures unlocks several key advantages, especially for delicate products.
Preserving Product Integrity
Many materials cannot tolerate high heat. Pharmaceuticals can lose their efficacy, plastics can warp, and some food products can lose nutritional value or flavor.
Vacuum drying allows for the safe processing of these materials, ensuring the final product maintains its intended physical and chemical properties.
Enhancing Drying Speed
The significant pressure difference between the product's interior and the vacuum chamber creates a strong driving force. This gradient pulls moisture from the core of the material to the surface much more effectively than heat alone.
Ensuring Uniform Drying
In conventional ovens, the outside of a product often dries much faster than the inside. The vacuum environment promotes a more uniform and consistent drying process throughout the entire product volume.
Understanding the Trade-offs: Vacuum Drying vs. Freeze Drying
While both use vacuums, it is critical to understand that vacuum drying and freeze drying (lyophilization) are not the same process and serve different goals.
The Goal of Standard Vacuum Drying
This process removes liquid water at low temperatures. It is ideal for materials like certain polymers, resins, minerals, and less delicate pharmaceuticals where the primary goal is simply to remove moisture without causing heat damage.
The Goal of Freeze Drying (Lyophilization)
Freeze drying is a more specialized, three-step process where the product is first frozen solid. The vacuum is then applied, causing the frozen water to turn directly into vapor without ever becoming a liquid (a process called sublimation).
This method is unmatched for preserving the delicate physical structure of a product, such as biological samples, vaccines, or high-end instant coffee. It preserves biological activity and allows for easy reconstitution with a solvent later.
Key Differences in Application
Use standard vacuum drying when your goal is to dry a heat-sensitive solid or powder. Use freeze drying when you must preserve the intricate structure and biological viability of the most delicate materials.
Making the Right Choice for Your Goal
Selecting the correct drying method depends entirely on the sensitivity of your material and your desired outcome.
- If your primary focus is drying heat-sensitive materials without altering their basic physical form: Standard vacuum drying is the most effective and efficient choice.
- If your primary focus is preserving the biological activity and delicate cellular structure of a product for later reconstitution: Freeze drying is the necessary and superior method.
- If your primary focus is bulk drying of non-sensitive, resilient materials where cost is the main driver: Conventional high-heat drying may be the most economical solution.
Ultimately, your choice is dictated by a simple question: how much damage can my product tolerate?
Summary Table:
| Benefit | Key Advantage |
|---|---|
| Gentle Drying | Prevents heat damage to sensitive materials like pharmaceuticals and plastics. |
| Faster Process | Lower pressure creates a strong driving force for efficient moisture removal. |
| Uniform Results | Promotes consistent drying throughout the entire product volume. |
| Oxidation Protection | Inert environment safeguards oxygen-sensitive products from degradation. |
Need to dry sensitive materials without compromising quality? KINTEK specializes in precision lab equipment, including vacuum drying solutions tailored for pharmaceuticals, polymers, and food products. Our expertise ensures you get the right equipment to preserve product integrity and enhance efficiency. Contact our experts today to discuss your specific drying requirements!
Visual Guide
Related Products
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Laboratory Benchtop Water Circulating Vacuum Pump for Lab Use
- Oil Free Diaphragm Vacuum Pump for Laboratory and Industrial Use
- High Performance Laboratory Freeze Dryer
- High Performance Laboratory Freeze Dryer for Research and Development
People Also Ask
- What is a sputtering machine? A Guide to High-Quality Thin Film Deposition
- Why is sintering easier in the presence of a liquid phase? Unlock Faster, Lower-Temperature Densification
- What is a vacuum furnace? The Ultimate Guide to Contamination-Free Thermal Processing
- How does a sputtering machine work? Achieve Atomic-Level Precision for Your Coatings
- What is a magnetron sputtering? A Guide to High-Quality Thin-Film Deposition



















