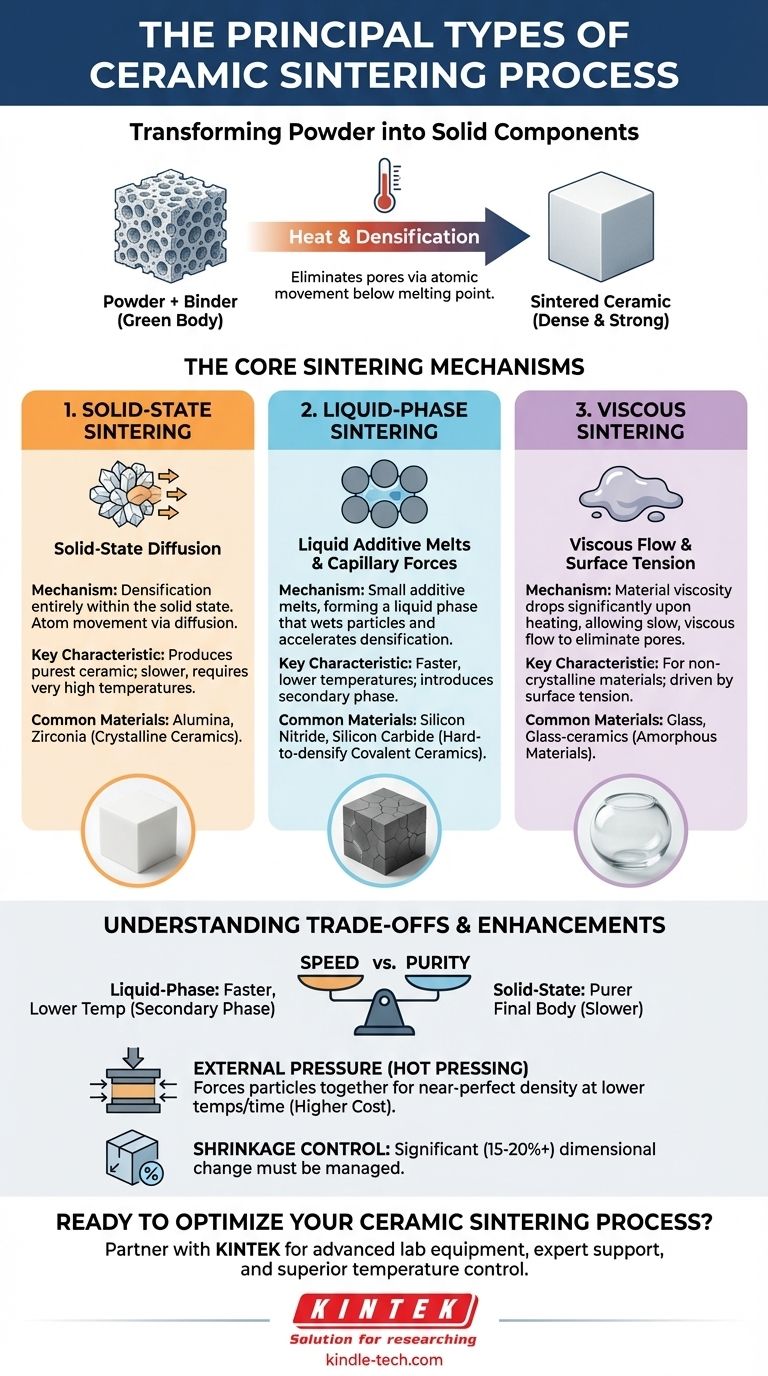
The principal types of ceramic sintering are Solid-State, Liquid-Phase, and Viscous Sintering. Each method uses a different physical mechanism to fuse ceramic powder into a dense, solid object. The choice of process is not arbitrary; it is dictated by the intrinsic properties of the ceramic material itself and the desired density of the final component.
The core challenge in sintering is getting solid particles to fuse together. The various sintering methods are simply different strategies for encouraging atoms to move and eliminate the empty spaces between those particles, with the choice of strategy depending entirely on the material's chemistry and melting behavior.

The Purpose of Sintering: From Powder to Solid
Before sintering can begin, a component must be formed. This is typically done by mixing ceramic powder with a binder and pressing it into a mold.
The resulting fragile part is called a "green body." It has the right shape but is highly porous and lacks mechanical strength. The fundamental goal of sintering is to heat this green body to a high temperature—below its melting point—to eliminate these pores and create a dense, strong ceramic.
How Sintering Works
During heating, atoms move from the contact points of the powder particles to fill the voids, or pores, between them. This process, driven by the reduction of surface energy, causes the particles to fuse together and the entire component to shrink and densify.
The specific mechanism by which the atoms move defines the type of sintering process.
The Core Sintering Mechanisms
The three primary mechanisms correspond to the three main types of sintering. Each is suited for a different class of ceramic material.
Solid-State Sintering
Solid-State Sintering is a process where densification occurs entirely while the material remains solid. Atom movement is achieved through solid-state diffusion.
This method is the "conventional" approach used for many common crystalline ceramics, such as alumina and zirconia. Because it relies solely on diffusion within a solid, it can be a slower process requiring very high temperatures.
Liquid-Phase Sintering
Liquid-Phase Sintering is used for materials that are very difficult to densify via solid-state diffusion alone. This includes high-performance ceramics like silicon nitride and silicon carbide.
In this process, small amounts of an additive are mixed with the ceramic powder. At sintering temperature, this additive melts and forms a liquid phase that wets the solid ceramic particles. This liquid accelerates densification dramatically through capillary forces, which pull the solid particles together and provide a fast path for material transport.
Viscous Sintering
Viscous Sintering applies specifically to amorphous, or non-crystalline, materials like glass.
Instead of distinct particles fusing, the material heats up, and its viscosity drops significantly. It behaves like a very thick liquid, and the pores are eliminated by slow, viscous flow under the force of surface tension.
Understanding the Trade-offs
Choosing a sintering process involves balancing speed, purity, and the final properties of the component.
Speed vs. Purity
Liquid-phase sintering is significantly faster and can be performed at lower temperatures than solid-state sintering.
However, it introduces an additive that becomes a permanent part of the final microstructure. While often beneficial, this secondary phase can sometimes compromise the material's performance at extreme temperatures or in corrosive environments. Solid-state sintering produces a purer final body.
The Role of External Pressure
For applications demanding near-perfect density, external pressure can be applied during heating. This technique is known as hot pressing.
Hot pressing physically forces the particles together, aiding densification and allowing for lower sintering temperatures or shorter times. It is a modification that can be applied to solid-state or liquid-phase processes but adds significant cost and complexity.
Shrinkage and Dimensional Control
All sintering processes cause the component to shrink as pores are eliminated. This shrinkage can be significant—often 15-20% or more.
This dimensional change must be precisely predicted and controlled. Factors like higher sintering temperatures or the presence of a liquid phase can affect the final amount of shrinkage and must be carefully managed to produce parts with accurate dimensions.
Making the Right Choice for Your Material
Your choice is determined almost entirely by the material you need to densify. The goal is to select the process whose mechanism is compatible with your material's fundamental properties.
- If your primary focus is a pure crystalline oxide (e.g., Alumina, Zirconia): Solid-state sintering is the standard and most effective approach.
- If your primary focus is a hard-to-densify covalent ceramic (e.g., Silicon Carbide, Silicon Nitride): Liquid-phase sintering is almost always required to achieve high density efficiently.
- If your primary focus is an amorphous material (e.g., glass or glass-ceramics): The densification will be governed by the principles of viscous sintering.
Understanding these core mechanisms is the key to successfully engineering and manufacturing high-performance ceramic components.
Summary Table:
| Sintering Type | Key Mechanism | Common Materials | Key Characteristic |
|---|---|---|---|
| Solid-State Sintering | Solid-state diffusion | Alumina, Zirconia | Produces pure ceramic; slower, high temperatures |
| Liquid-Phase Sintering | Liquid phase accelerates densification | Silicon Nitride, Silicon Carbide | Faster; introduces secondary phase |
| Viscous Sintering | Viscous flow of amorphous material | Glass, Glass-ceramics | For non-crystalline materials; driven by surface tension |
Ready to Optimize Your Ceramic Sintering Process?
Choosing the right sintering method is critical for achieving the density, purity, and performance your application demands. The experts at KINTEK are here to help. We specialize in providing the advanced lab equipment and consumables necessary for precise thermal processing, from research and development to full-scale production.
Partner with KINTEK to:
- Select the ideal furnace technology for your specific ceramic material and sintering process.
- Achieve superior temperature control and uniformity for consistent, high-quality results.
- Access expert technical support to troubleshoot challenges and optimize your sintering parameters.
Don't let sintering complexities slow down your innovation. Contact our thermal processing specialists today to discuss your project needs and discover how KINTEK's solutions can enhance your laboratory's capabilities.
Visual Guide
Related Products
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
People Also Ask
- What materials are used for the tubes in tube furnaces? A Guide to Selecting the Right Tube for Your Process
- How to clean a tube furnace? A Step-by-Step Guide for Safe and Effective Maintenance
- What precautions should be taken when using a tube furnace? Ensure Safe, Effective High-Temperature Processing
- How does a quartz tube vacuum furnace contribute to the crystallization process of Ag-doped Li-argyrodite electrolytes?
- What is a tubular furnace used for? Precision Heating for Material Synthesis & Analysis



















