At its core, the five-port water bath electrolytic cell is a specialized tool designed for control and versatility in electrochemical experiments. Its key features include a double-walled glass body for precise temperature control via a circulating water bath, five access ports for flexible configuration of electrodes and probes, support for a Luggin capillary to ensure accurate measurements, and provisions for controlling the gaseous atmosphere within the cell.
This cell is more than just a container; it is a highly controlled micro-environment. Its design prioritizes isolating and managing the key variables of an experiment—temperature, potential, and atmosphere—to deliver reproducible and accurate electrochemical data.

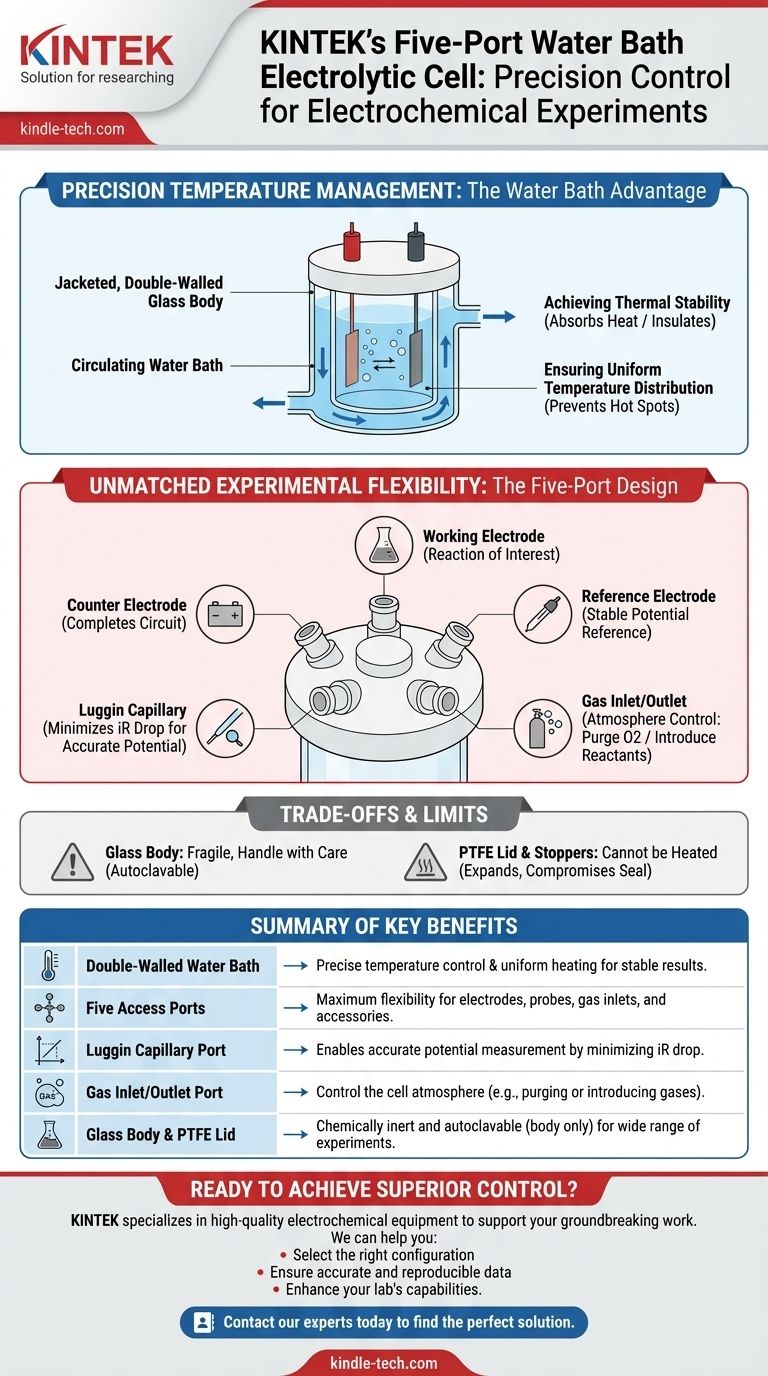
Precision Temperature Management: The Water Bath Advantage
A defining feature of this cell is the jacketed, double-walled glass body. This allows a temperature-controlled liquid, typically water, to be circulated around the inner reaction chamber.
Achieving Thermal Stability
The circulating water bath acts as a large thermal sink or source. It effectively absorbs excess heat generated by the electrochemical reaction or insulates the cell from fluctuations in ambient room temperature.
This stability is critical for studying temperature-dependent phenomena, ensuring that thermal drift does not become a hidden variable in your results.
Ensuring Uniform Temperature Distribution
The water jacket ensures the temperature is consistent across the entire cell. This prevents localized "hot spots" on the electrode surfaces, which can alter reaction rates and lead to inconsistent data.
Uniform distribution is essential for achieving reliable measurements in kinetics, catalysis, and electrodeposition studies.
Enabling Temperature-Sensitive Studies
Many electrochemical processes, such as organic electrosynthesis or battery material analysis, are highly sensitive to temperature. This cell provides the stable, controlled thermal environment required for such work.
Unmatched Experimental Flexibility: The Five-Port Design
The five ports in the cell's lid (typically made of PTFE) are the key to its adaptability. They allow the researcher to build a custom setup tailored to the specific experiment.
The Standard Electrode Configuration
Three ports are typically used for the standard three-electrode setup: the working electrode (where the reaction of interest occurs), the counter electrode (which completes the circuit), and the reference electrode (which provides a stable potential reference).
The Critical Role of the Luggin Capillary
One port is specifically designed to accommodate a Luggin capillary. This thin tube allows the tip of the reference electrode to be placed very close to the working electrode surface.
Its purpose is to minimize the iR drop—an error in potential measurement caused by the resistance of the electrolyte. Using a Luggin capillary is essential for accurate measurements, especially in solutions with low conductivity.
Controlling the Gaseous Atmosphere
Another port is used for a gas inlet/outlet tube. This allows you to purge the electrolyte with an inert gas like nitrogen or argon to remove dissolved oxygen, which can interfere with many reactions.
Alternatively, it can be used to introduce a specific reactant gas, such as CO2, into the solution for studies like electrochemical reduction.
Advanced Techniques and Accessories
The port design is often compatible with specialized equipment like a rotating disk electrode (RDE) for hydrodynamic studies or electrode extension rods for custom positioning.
Understanding the Trade-offs and Practical Limits
While powerful, this cell has practical constraints that every user must understand to ensure safety and data integrity.
Material Constraints: Glass and PTFE
The cell body is made of glass, making it fragile. It must always be handled with care to prevent breakage.
While the glass body can be sterilized via autoclaving (e.g., at 121°C), the Polytetrafluoroethylene (PTFE) lid and stoppers cannot be heated. PTFE expands significantly when heated and may not return to its original shape, compromising the cell's seal.
Assembly and Sealing
Proper assembly is crucial. The standard configuration often includes a liquid seal device and PTFE stoppers to ensure the system is airtight. A poor seal can lead to contamination from the ambient atmosphere.
Making the Right Choice for Your Research
To get the most out of this cell, configure it to address the primary challenge of your specific experiment.
- If your primary focus is kinetic or thermodynamic studies: The water bath feature is non-negotiable for achieving the required temperature stability.
- If your primary focus is corrosion or high-impedance media: Using the Luggin capillary is critical to obtaining an accurate measurement of the electrode potential.
- If your primary focus is oxygen-sensitive reactions: Utilize the gas inlet port to thoroughly purge the electrolyte with an inert gas before and during your experiment.
- If your primary focus is mass-transport phenomena: Ensure your setup is compatible with a rotating disk electrode (RDE) to control the convective flux to your electrode.
Ultimately, mastering this cell is about leveraging its modularity to systematically control the variables that matter most in your experiment.
Summary Table:
| Feature | Key Benefit |
|---|---|
| Double-Walled Water Bath | Precise temperature control & uniform heating for stable, reproducible results. |
| Five Access Ports | Maximum flexibility for electrodes, probes, gas inlets, and specialized accessories. |
| Luggin Capillary Port | Enables accurate potential measurement by minimizing iR drop in the electrolyte. |
| Gas Inlet/Outlet Port | Control the cell atmosphere (e.g., purging oxygen or introducing reactant gases). |
| Glass Body & PTFE Lid | Chemically inert and autoclavable (body only) for a wide range of experiments. |
Ready to achieve superior control in your electrochemical research?
The five-port water bath electrolytic cell is engineered for researchers who demand precision and versatility. At KINTEK, we specialize in providing high-quality lab equipment, including electrochemical cells and accessories, to support your groundbreaking work.
We can help you:
- Select the right configuration for your specific application, from kinetics to corrosion studies.
- Ensure accurate and reproducible data by controlling temperature, potential, and atmosphere.
- Enhance your lab's capabilities with reliable equipment designed for rigorous experimental demands.
Let's discuss your project requirements. Contact our experts today to find the perfect solution for your laboratory needs.
Visual Guide
Related Products
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Multifunctional Electrolytic Electrochemical Cell Water Bath Single Layer Double Layer
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- Optical Water Bath Electrolytic Electrochemical Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
People Also Ask
- What is the overall structure of the H-type double-layer optical water bath electrolytic cell? Precision Design for Controlled Experiments
- When is professional repair required for a double-layer water-bath electrolytic cell? Protect Your Lab's Precision and Safety
- What are the key features of a double-layer water-bath electrolytic cell? Achieve Precise Temperature Control for Your Experiments
- What is the typical experimental system used with a double-layer water-bath electrolytic cell? Achieve Precise Electrochemical Control
- How can the electrochemical reaction be controlled when using this electrolytic cell? Master Voltage, Current & Electrolyte



















