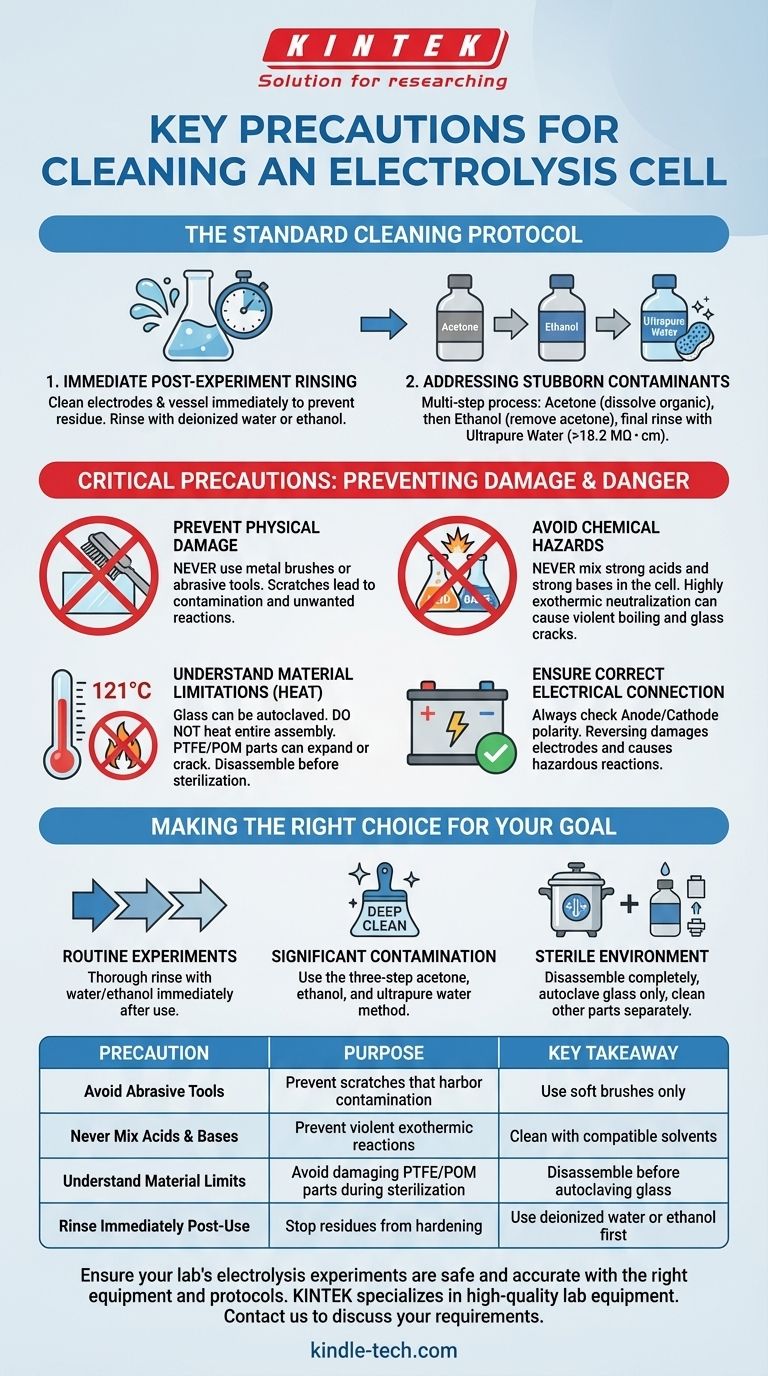
Properly cleaning an electrolysis cell is fundamental to ensuring both experimental accuracy and laboratory safety. The key precautions involve avoiding physical damage with abrasive tools, preventing dangerous chemical reactions by never mixing acids and bases, and understanding the material limitations of the cell's components, especially concerning heat.
The core principle of cleaning an electrolysis cell is to remove all contaminants from a previous experiment without introducing new ones or damaging the apparatus. This requires a methodical approach that respects the chemical and physical properties of the cell's materials.

The Standard Cleaning Protocol
A consistent cleaning process is the first line of defense against contamination and degradation. This should be performed immediately after an experiment concludes.
Immediate Post-Experiment Rinsing
The most critical step is to clean the electrodes and reaction vessel immediately after use. This prevents residues from drying and adhering to the surfaces.
For routine cleaning, a simple rinse with deionized water or ethanol is often sufficient to remove residual electrolytes and reaction products.
Addressing Stubborn Contaminants
If residues persist, a more thorough multi-step process is required. This ensures all organic and inorganic materials are systematically removed.
First, scrub the inner walls with acetone to dissolve organic compounds. Follow this with an ethanol rinse to remove the acetone.
The final step is a thorough rinse with ultrapure water (resistivity >18.2 MΩ・cm) to eliminate any remaining solvents and inorganic ions.
Critical Precautions to Prevent Damage and Danger
Beyond the cleaning sequence, several absolute rules must be followed to protect the equipment and the operator. Ignoring these can lead to costly damage or serious injury.
Preventing Physical Damage
Never use metal brushes or other hard, abrasive tools to clean the cell.
These implements will inevitably scratch the glass and electrode surfaces. Scratches are not merely cosmetic; they increase the surface area in an uncontrolled way and can become sites for contamination or unwanted side reactions, compromising future results.
Avoiding Chemical Hazards
Under no circumstances should you mix strong acids (e.g., HNO₃) and strong bases (e.g., NaOH) within the cell for cleaning purposes.
This combination triggers a highly exothermic neutralization reaction, which can generate significant heat very quickly. This can cause the solution to boil violently, splashing corrosive chemicals, or even crack the glass cell due to thermal shock.
Understanding Material Limitations with Heat
While the glass body of the cell can typically be sterilized in an autoclave at 121℃, you must not heat the entire cell assembly.
Components made from PTFE (Teflon) can expand permanently when heated, preventing a proper seal. Parts made from POM (polyoxymethylene) are prone to cracking under high heat stress. Always disassemble the cell before sterilizing any glass components.
Ensuring Correct Electrical Connection
Though not a cleaning step, it is a critical handling precaution. Always ensure the anode and cathode are connected correctly to the power source.
Reversing the polarity can damage the electrodes or lead to unintended, potentially hazardous chemical reactions, ruining your experiment and equipment.
Making the Right Choice for Your Goal
Your cleaning strategy should match the needs of your experiment.
- If your primary focus is routine, sequential experiments: A thorough rinse with deionized water or ethanol immediately after use is the most effective practice.
- If your primary focus is removing significant contamination: Use the three-step acetone, ethanol, and ultrapure water method to restore the cell to a pristine state.
- If your primary focus is working in a sterile environment: Disassemble the unit completely and only sterilize the glass components via autoclave, cleaning all other parts separately.
Following these disciplined procedures will protect your equipment, ensure your safety, and produce reliable, reproducible results.
Summary Table:
| Precautions | Purpose | Key Takeaway |
|---|---|---|
| Avoid Abrasive Tools | Prevent scratches that harbor contamination | Use soft brushes only |
| Never Mix Acids & Bases | Prevent violent exothermic reactions | Clean with compatible solvents |
| Understand Material Limits | Avoid damaging PTFE/POM parts during sterilization | Disassemble before autoclaving glass |
| Rinse Immediately Post-Use | Stop residues from hardening | Use deionized water or ethanol first |
Ensure your lab's electrolysis experiments are safe and accurate with the right equipment and protocols. KINTEK specializes in high-quality lab equipment and consumables, including durable electrolysis cells designed for easy cleaning and long-term reliability. Our experts can help you select the perfect setup for your specific needs, ensuring optimal performance and safety. Contact us today to discuss your laboratory requirements and discover how KINTEK can support your research with precision and care.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell with Five-Port
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Optical Water Bath Electrolytic Electrochemical Cell
People Also Ask
- What types of electrodes are used in the H-type electrolytic cell? Expert Guide to Three-Electrode Systems
- What safety precautions should be taken during an experiment with the electrolytic cell? A Guide to Preventing Shocks, Burns, and Fires
- What are the core functions of specialized photoelectrochemical electrolytic cells in HER? Precision Evaluation for Lab
- How should the experimental parameters be adjusted when using the H-type electrolytic cell? Expert Precision Guide
- What are the structural characteristics and advantages of the all-PTFE electrolytic cell? Ultimate Chemical Inertness
- Why is a glass electrochemical cell with a plexiglass lid used for Zr2.5Nb alloys? Ensure Precision in Corrosion Tests
- Why is Polyetheretherketone (PEEK) chosen for XAS electrochemical cells? Ensure Chemical Inertness and Precision
- How does the design of an electrolytic cell influence evaluation of electrochemical catalytic performance? Key Factors



















