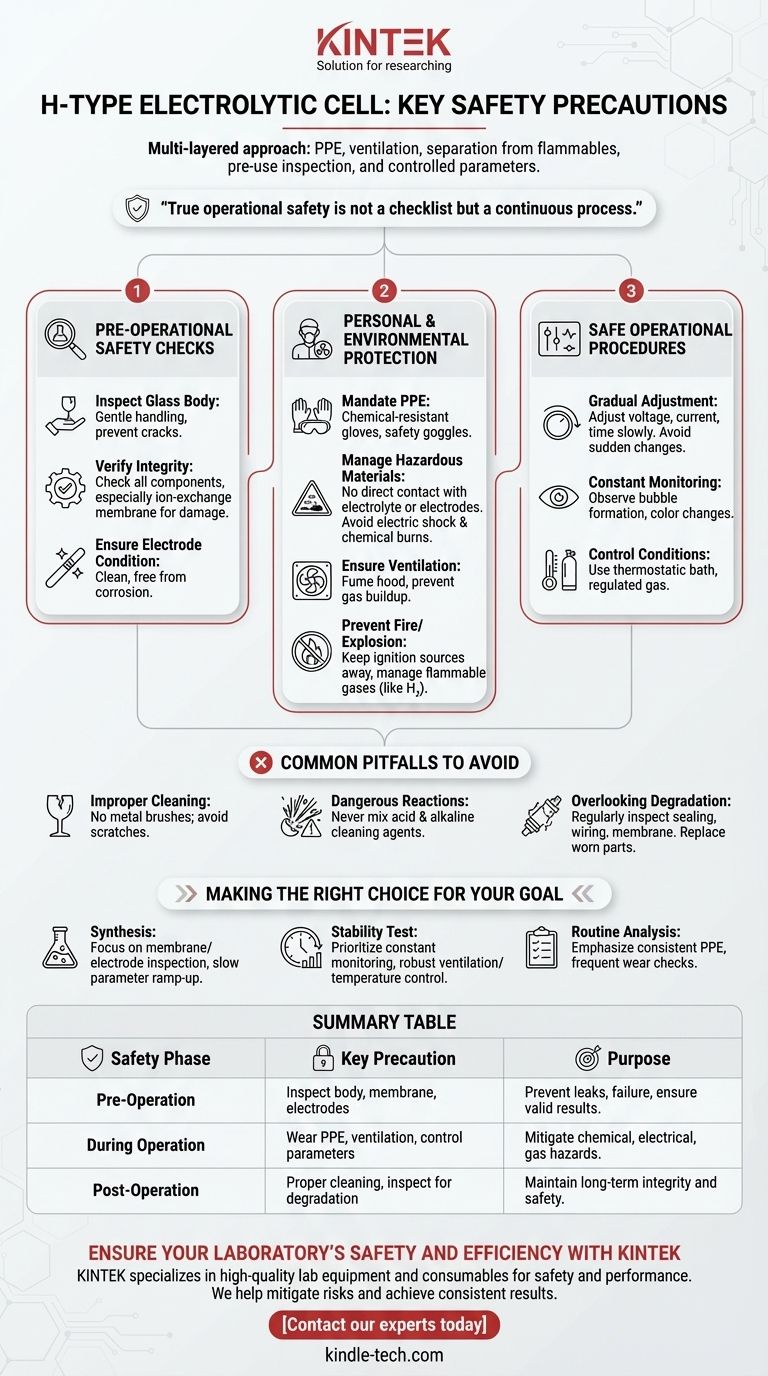
The most critical safety precautions for operating an H-type electrolytic cell involve a multi-layered approach. You must use personal protective equipment (PPE) like gloves and goggles, ensure proper ventilation to manage hazardous gases, and maintain a strict separation from flammable materials. Safe operation also demands careful pre-use inspection of all components and methodical control of electrical parameters during the experiment.
True operational safety is not a checklist but a continuous process. It begins with a thorough inspection before the experiment, extends through diligent monitoring during operation, and concludes with proper cleaning and maintenance.

Pre-Operational Safety Checks
Before any power is applied, a meticulous inspection is the foundation of a safe experiment. This step prevents equipment failure that can lead to chemical or electrical hazards.
Inspecting the Glass Cell Body
The main body of an H-type cell is typically made of glass, which is fragile. Handle it gently at all times to prevent cracks or breakage that could lead to dangerous leaks of electrolyte.
Verifying Component Integrity
Confirm that all components are present and in good condition. This includes the anode and cathode chambers, lids, and sealing rings. Most importantly, check that the ion-exchange membrane is intact, with no visible damage or signs of aging.
Ensuring Electrode Condition
The surfaces of the electrodes must be clean and free from corrosion or physical damage. If necessary, clean or polish the electrodes before assembly to ensure both safety and the validity of your experimental results.
Personal and Environmental Protection
Your immediate safety and the stability of the lab environment are paramount. These precautions mitigate the primary risks of chemical, electrical, and fire hazards.
Mandating Personal Protective Equipment (PPE)
Always wear appropriate PPE. This includes chemical-resistant gloves to protect from the electrolyte and safety goggles to prevent splashes from contacting your eyes.
Managing Hazardous Materials
Never make direct contact with the electrodes or the electrolyte solution. This prevents the dual risks of electric shock from the power supply and chemical burns from corrosive materials.
Ensuring Proper Ventilation
The electrolysis process can produce harmful or flammable gases. Conduct your experiment in a well-ventilated area or under a fume hood to prevent the buildup of a dangerous atmosphere.
Preventing Fire and Explosion Hazards
Some electrolytic processes generate flammable gases like hydrogen. It is absolutely essential to keep open flames, sparks, and other ignition sources far away from the cell's vicinity to prevent fire or explosion.
Safe Operational Procedures
During the experiment, safe practices revolve around control and observation. Abrupt changes or unmonitored operation can quickly lead to unsafe conditions.
Gradual Adjustment of Parameters
When you begin the experiment, adjust the voltage, current, and electrolysis time gradually. Sudden changes can cause unexpected reactions or damage the cell components.
Constant Monitoring
Closely observe the working state of the cell. Look for key indicators like bubble formation on the electrode surfaces or any color changes in the electrolyte. These signs can alert you to potential issues that require immediate attention.
Controlling Experimental Conditions
If your experiment requires specific conditions, ensure they are properly managed. Use a thermostatic water bath for constant temperature and a regulated gas cylinder if a specific atmosphere, like nitrogen, is needed.
Common Pitfalls to Avoid
Even experienced operators can make mistakes. Being aware of these common errors is crucial for maintaining a safe laboratory environment.
The Risk of Improper Cleaning
Never use metal brushes to clean the cell, as they can scratch and weaken the glass surfaces. A scratched cell is more prone to breaking under thermal or physical stress.
Dangerous Cleaning Agent Reactions
It is strictly forbidden to mix acid and alkaline cleaning agents, such as nitric acid and sodium hydroxide. This combination can cause a violent and dangerous exothermic reaction.
Overlooking Component Degradation
Safety is an ongoing concern. Regularly inspect the cell's sealing integrity and check for signs of aging in the wiring or membrane. Components degrade over time and must be replaced before they fail.
Making the Right Choice for Your Goal
Your specific experimental goal should inform your primary safety focus.
- If your primary focus is synthesizing a new material: Pay extra attention to the pre-operational inspection of the membrane and electrodes, and ramp up electrical parameters very slowly.
- If your primary focus is a long-duration stability test: Prioritize constant monitoring of the cell's state and ensure your ventilation and temperature controls are robust and reliable.
- If your primary focus is routine analysis or teaching: Emphasize consistent use of PPE and conduct frequent checks for component wear to avoid complacency-driven accidents.
Ultimately, a proactive and knowledgeable approach is your best defense against accidents.
Summary Table:
| Safety Phase | Key Precaution | Purpose |
|---|---|---|
| Pre-Operation | Inspect glass body, membrane, and electrodes | Prevent leaks, equipment failure, and ensure valid results |
| During Operation | Wear PPE, ensure ventilation, control parameters gradually | Mitigate chemical, electrical, and gas hazards |
| Post-Operation | Use proper cleaning methods and inspect for degradation | Maintain long-term equipment integrity and safety |
Ensure Your Laboratory's Safety and Efficiency with KINTEK
Operating specialized equipment like H-type electrolytic cells demands precision and the highest safety standards. Whether you are synthesizing new materials, running stability tests, or conducting routine analyses, having reliable equipment is non-negotiable.
KINTEK specializes in providing high-quality lab equipment and consumables designed for safety and performance. We understand the critical needs of laboratories, from durable glassware and intact ion-exchange membranes to stable power supplies.
Let us help you mitigate risks and achieve consistent, accurate results. Our products are selected to meet the rigorous demands of your research, ensuring that every precaution you take is supported by dependable tools.
Contact our experts today to find the right equipment for your electrolysis experiments and enhance your lab's safety protocol.
Visual Guide
Related Products
- H Type Electrolytic Cell Triple Electrochemical Cell
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell with Five-Port
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
People Also Ask
- What are the proper storage conditions for an H-type electrolytic cell? Ensure Long-Term Reliability and Accurate Results
- What preparation steps are needed before starting an experiment with an H-type electrolytic cell? A Guide to Safe and Accurate Results
- How should the H-type electrolytic cell be stored when not in use? Expert Storage & Maintenance Guide
- What are the key safety operation guidelines for using the H-type electrolytic cell? Best Practices for Your Lab
- What is the purpose of the double-layer structure in the H-type electrolytic cell? Achieve Precise Thermal Control



















