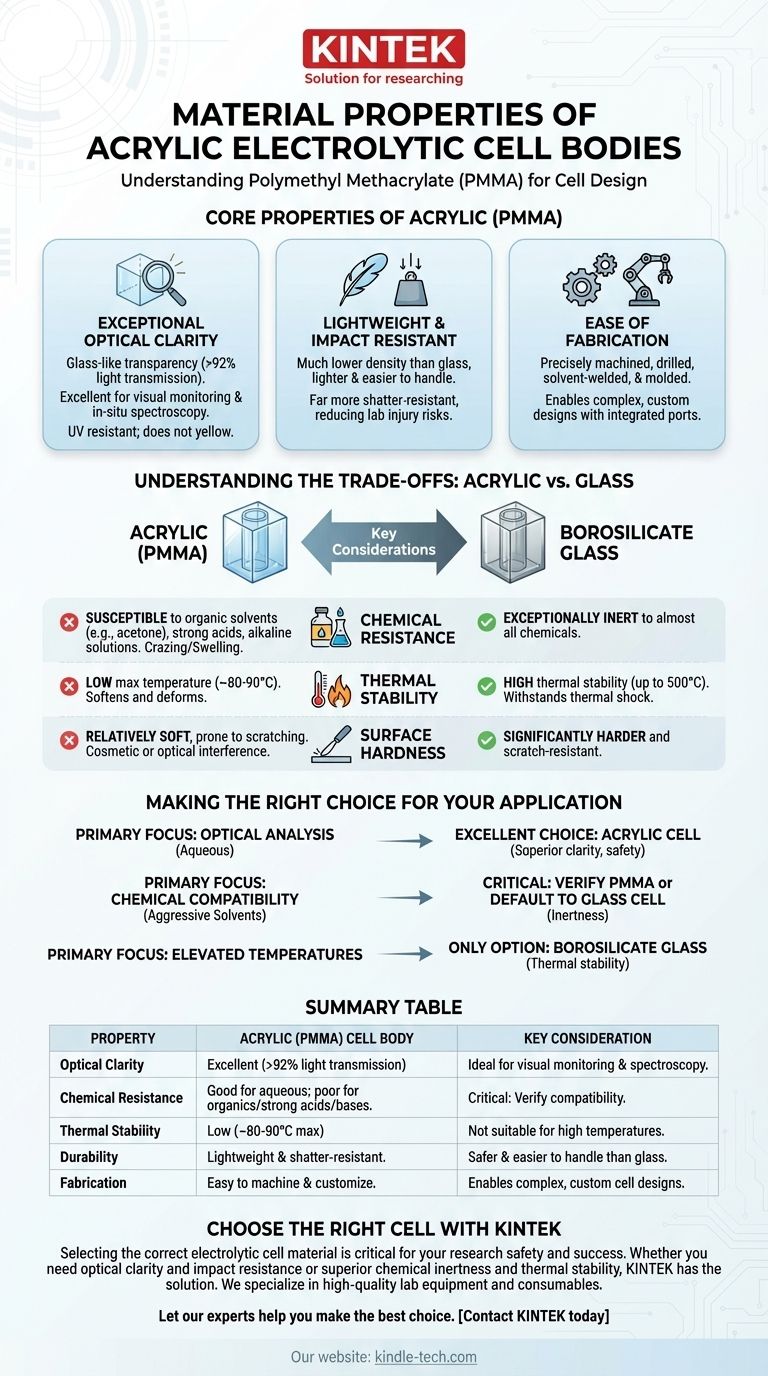
To understand its performance, an acrylic electrolytic cell body is made from Polymethyl Methacrylate (PMMA). This specific polymer is chosen for its unique combination of high optical transparency, lightweight nature, ease of processing into specific shapes, and excellent long-term resistance to yellowing from ultraviolet (UV) radiation.
Acrylic is selected for electrolytic cells when visual clarity and ease of fabrication are the highest priorities. However, its suitability is entirely dependent on its chemical compatibility with the electrolyte, representing its most significant trade-off compared to materials like glass.

Core Properties of Acrylic (PMMA) in Cell Design
Exceptional Optical Clarity
The primary advantage of PMMA is its glass-like transparency, often exceeding 92% light transmission. This is critical for applications requiring visual monitoring of electrode processes or in-situ spectroscopic analysis.
Its inherent resistance to UV radiation means it will not yellow or become brittle over time when exposed to sunlight or other UV sources, preserving optical integrity for long-term experiments.
Lightweight and Impact Resistant
PMMA has a much lower density than glass, making cells significantly lighter and easier to handle, especially in larger configurations.
While not unbreakable, acrylic is far more shatter-resistant than glass. This reduces the risk of injury and catastrophic failure from accidental impacts in a lab environment.
Ease of Fabrication
The term "easy to process" means PMMA can be precisely machined, drilled, solvent-welded, and molded.
This allows for the creation of complex and custom cell designs with integrated ports for electrodes, sensors, and fluid flow, which can be difficult or expensive to achieve with glass.
Understanding the Trade-offs: Acrylic vs. Glass
While acrylic offers many benefits, it is not a universal solution. Understanding its limitations, particularly when compared to borosilicate glass, is essential for experimental success.
Chemical Resistance
This is the most critical trade-off. Acrylic has good resistance to many aqueous solutions, salts, and weak acids.
However, it is highly susceptible to attack from organic solvents (like acetone and alcohols), strong acids, and alkaline solutions. This exposure can cause crazing (micro-fractures), swelling, or complete dissolution, compromising the cell's integrity. Borosilicate glass, in contrast, is exceptionally inert to almost all chemicals.
Thermal Stability
Acrylic has a low maximum operating temperature, typically around 80-90°C, above which it begins to soften and deform.
Borosilicate glass can withstand thermal shock and is stable at much higher temperatures (up to 500°C), making it the only choice for high-temperature electrolysis.
Surface Hardness
The surface of acrylic is relatively soft and prone to scratching from abrasive materials or cleaning. While this may only be a cosmetic issue, deep scratches can interfere with optical measurements. Glass is significantly harder and more scratch-resistant.
Making the Right Choice for Your Application
- If your primary focus is optical analysis in aqueous solutions: An acrylic (PMMA) cell is an excellent choice, offering superior clarity and safety from breakage.
- If your primary focus is chemical compatibility with aggressive solvents: You must verify PMMA's resistance to your specific chemicals or default to a borosilicate glass cell for its superior inertness.
- If your experiment involves elevated temperatures: Borosilicate glass is the only appropriate option due to acrylic's low thermal stability.
Ultimately, choosing the correct cell material is a foundational decision that directly impacts the safety, accuracy, and validity of your electrochemical experiments.
Summary Table:
| Property | Acrylic (PMMA) Cell Body | Key Consideration |
|---|---|---|
| Optical Clarity | Excellent (>92% light transmission) | Ideal for visual monitoring and spectroscopy. |
| Chemical Resistance | Good for aqueous solutions; poor for organics, strong acids/bases. | Critical: Verify compatibility with your electrolyte. |
| Thermal Stability | Low (~80-90°C max) | Not suitable for high-temperature experiments. |
| Durability | Lightweight and shatter-resistant. | Safer and easier to handle than glass. |
| Fabrication | Easy to machine and customize. | Enables complex, custom cell designs. |
Choose the Right Cell for Your Experiment with KINTEK
Selecting the correct electrolytic cell material is critical for the safety and success of your research. Whether you need the optical clarity and impact resistance of acrylic or the superior chemical inertness and thermal stability of borosilicate glass, KINTEK has the solution.
We specialize in high-quality lab equipment and consumables, providing the right tools for your specific laboratory needs.
Let our experts help you make the best choice. Contact KINTEK today to discuss your application requirements!
Visual Guide
Related Products
- Electrolytic Electrochemical Cell with Five-Port
- Electrolytic Electrochemical Cell for Coating Evaluation
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
- Customizable PEM Electrolysis Cells for Diverse Research Applications
People Also Ask
- How can leaks be prevented when using a five-port water bath electrolytic cell? Ensure a Reliable and Safe Electrochemical Setup
- How can contamination be avoided during experiments with the five-port water bath electrolytic cell? Master the 3-Pillar Protocol
- What are the standard components of the five-port water bath electrolytic cell? Master the Precision Instrument for Electrochemical Analysis
- How should the body of an electrolytic cell be maintained for longevity? Extend Your Equipment's Lifespan
- What is the proper way to handle a five-port water bath electrolytic cell? Ensure Accurate and Safe Electrochemical Experiments



















