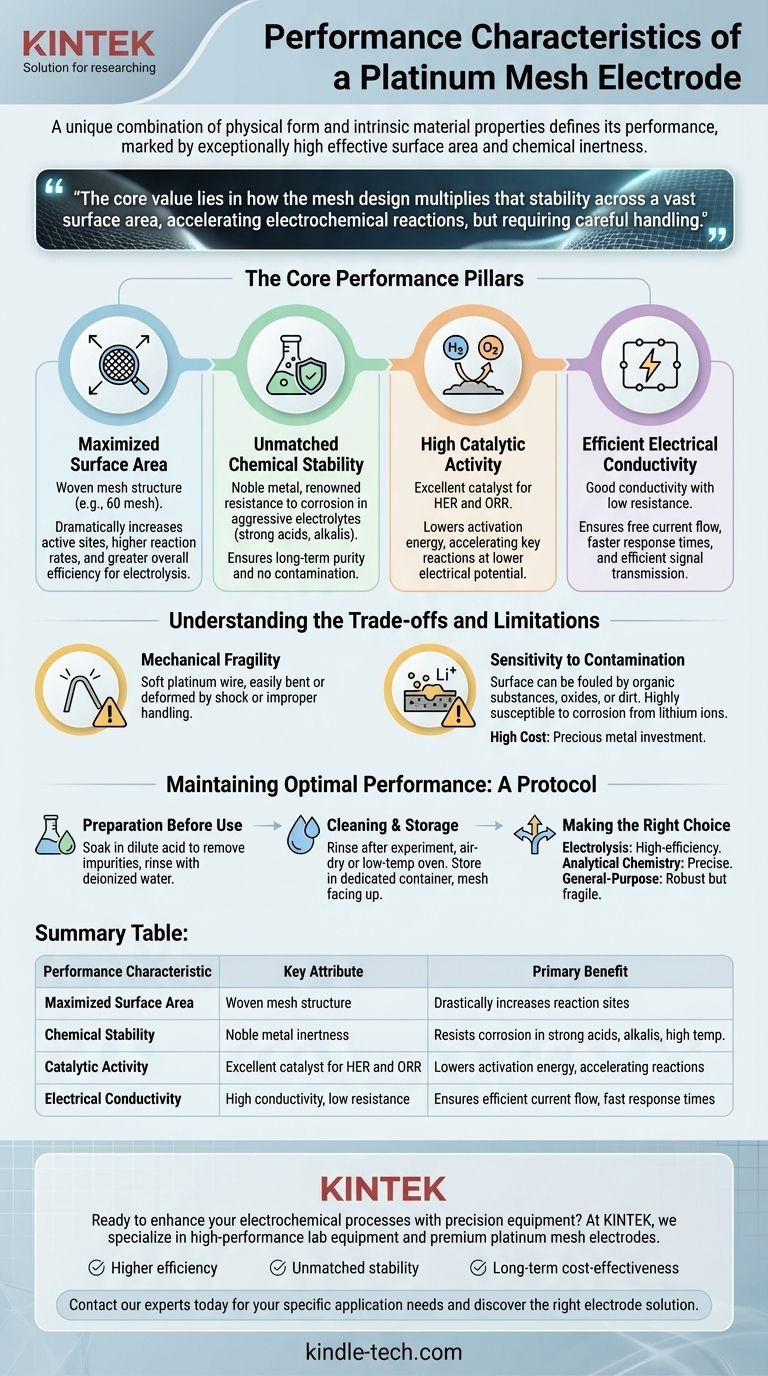
In electrochemical applications, a platinum mesh electrode's performance is defined by its unique combination of physical form and intrinsic material properties. It is characterized by an exceptionally high effective surface area due to its woven mesh structure, which enhances reaction efficiency. This is paired with platinum's inherent chemical inertness, high electrical conductivity, and strong catalytic activity, making it stable and effective in harsh conditions like strong acids, alkalis, and high temperatures.
The core value of a platinum mesh electrode lies not just in platinum's stability, but in how the mesh design multiplies that stability across a vast surface area. This makes it a powerful tool for accelerating electrochemical reactions, but one that requires careful handling to maintain its integrity.
The Core Performance Pillars
The performance of a platinum mesh electrode is built on four key characteristics that work in concert. Understanding each one is crucial to leveraging its full potential in an experimental setup.
Maximized Surface Area
The defining feature is its structure as a woven mesh, often specified by a measure like 60 mesh. This design dramatically increases the available surface area for reactions compared to a solid plate or wire of the same geometric dimensions.
More surface area means more active sites for electrochemical processes to occur simultaneously. This directly translates to higher reaction rates and greater overall efficiency for applications like electrolysis.
Unmatched Chemical Stability
Platinum is a noble metal, renowned for its resistance to corrosion and chemical attack. It remains stable and unreactive even when submerged in aggressive electrolytes, including strong acids (hydrochloric, sulfuric, nitric) and strong alkalis.
This inertness ensures that the electrode itself does not corrode, break down, or introduce contaminating ions into your solution. This quality is critical for long-term experiments and for maintaining the purity of your results.
High Catalytic Activity
Beyond just being a stable surface, platinum is an excellent catalyst for many important electrochemical reactions. It is particularly effective for the hydrogen evolution reaction (HER) and the oxygen reduction reaction (ORR).
This catalytic performance lowers the activation energy required for these reactions to proceed, meaning they can occur more efficiently and at a lower electrical potential.
Efficient Electrical Conductivity
Like all effective electrodes, platinum possesses good electrical conductivity. This property ensures that current can flow freely between the power source, the electrode, and the electrolyte with minimal resistive loss.
Low internal resistance contributes to faster response times and more efficient signal transmission, which is essential for both preparative and analytical electrochemical work.
Understanding the Trade-offs and Limitations
While powerful, a platinum mesh electrode is not without its drawbacks. Its unique properties come with specific vulnerabilities that must be managed.
Mechanical Fragility
The platinum wire used to create the mesh is soft and malleable. The electrode can be easily bent, deformed, or torn if subjected to mechanical shock, pressure, or improper handling.
This fragility contrasts with more robust electrode forms like solid rods or thick plates, requiring greater care during setup, cleaning, and storage.
Sensitivity to Contamination
The electrode's performance is highly dependent on a clean, active surface. The surface can be "fouled" or "poisoned" by contact with organic substances, oxides, or dirt, which increases its electrical resistance and blocks active sites.
Crucially, platinum is highly susceptible to corrosion from lithium ions. Contact with any lithium-containing compounds is strictly prohibited and can permanently damage the electrode.
High Cost
Platinum is a precious metal, and its high cost химиis a significant factor. While its longevity can justify the investment for critical applications, it represents a substantial upfront expense compared to other electrode materials.
Maintaining Optimal Performance: A Protocol
To ensure repeatable and accurate results, a strict handling and cleaning protocol is necessary.
Preparation Before Use
Before its first use or after long storage, soak the mesh in a dilute acid (such as dilute nitric acid) to remove any surface oxides or impurities. Rinse it thoroughly with deionized water afterward.
Cleaning and Storage
Immediately after an experiment, rinse the electrode with deionized water to remove all traces of electrolyte. Allow it to air-dry or place it in a low-temperature oven.
Store the clean, dry electrode in a dedicated container where it will not be contaminated. Place it with the mesh facing upwards to avoid putting stress on the connection point.
Making the Right Choice
Choosing an electrode requires matching its characteristics to your primary objective.
- If your primary focus is high-efficiency electrolysis: The massive surface area and catalytic activity of the mesh make it ideal for driving reactions like water splitting.
- If your primary focus is precise analytical chemistry: Its chemical inertness is a major advantage, but you must be exceptionally vigilant about cleaning to prevent surface fouling from skewing your measurements.
- If your primary focus is general-purpose, robust lab work: Its chemical durability is excellent, but its mechanical fragility and high cost may make a solid platinum or alternative material electrode a more practical choice.
Ultimately, mastering the use of a platinum mesh electrode is about leveraging its immense surface area while respecting its physical and chemical vulnerabilities.
Summary Table:
| Performance Characteristic | Key Attribute | Primary Benefit |
|---|---|---|
| Maximized Surface Area | Woven mesh structure (e.g., 60 mesh) | Drastically increases reaction sites for higher efficiency |
| Chemical Stability | Noble metal inertness | Resists corrosion in strong acids, alkalis, and high temperatures |
| Catalytic Activity | Excellent catalyst for HER and ORR | Lowers activation energy, accelerating key reactions |
| Electrical Conductivity | High conductivity with low resistance | Ensures efficient current flow and fast response times |
Ready to enhance your electrochemical processes with precision equipment?
At KINTEK, we specialize in providing high-performance lab equipment and consumables, including premium platinum mesh electrodes designed for reliability and superior results. Our products are trusted by laboratories worldwide for their quality and durability in demanding environments.
Let us help you achieve:
- Higher efficiency in electrolysis and catalytic reactions.
- Unmatched stability in aggressive chemical conditions.
- Long-term cost-effectiveness through robust, contamination-resistant materials.
Contact our experts today to discuss your specific application needs and discover the right electrode solution for your laboratory.
Visual Guide
Related Products
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Platinum Auxiliary Electrode for Laboratory Use
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Metal Disc Electrode Electrochemical Electrode
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
People Also Ask
- What is the most critical guideline for immersing a platinum sheet electrode in an electrolyte? Ensure Accurate Electrochemical Measurements
- How should a platinum sheet electrode be pretreated before use? Ensure Accurate Electrochemical Measurements
- What are the performance characteristics of platinum sheet electrodes? Unlock Superior Electrochemical Performance
- What are the key performance characteristics and applications of platinum sheets? Unmatched Reliability for Demanding Applications
- What is the expected lifespan of a platinum sheet electrode? Maximize Your Electrode's Service Life



















