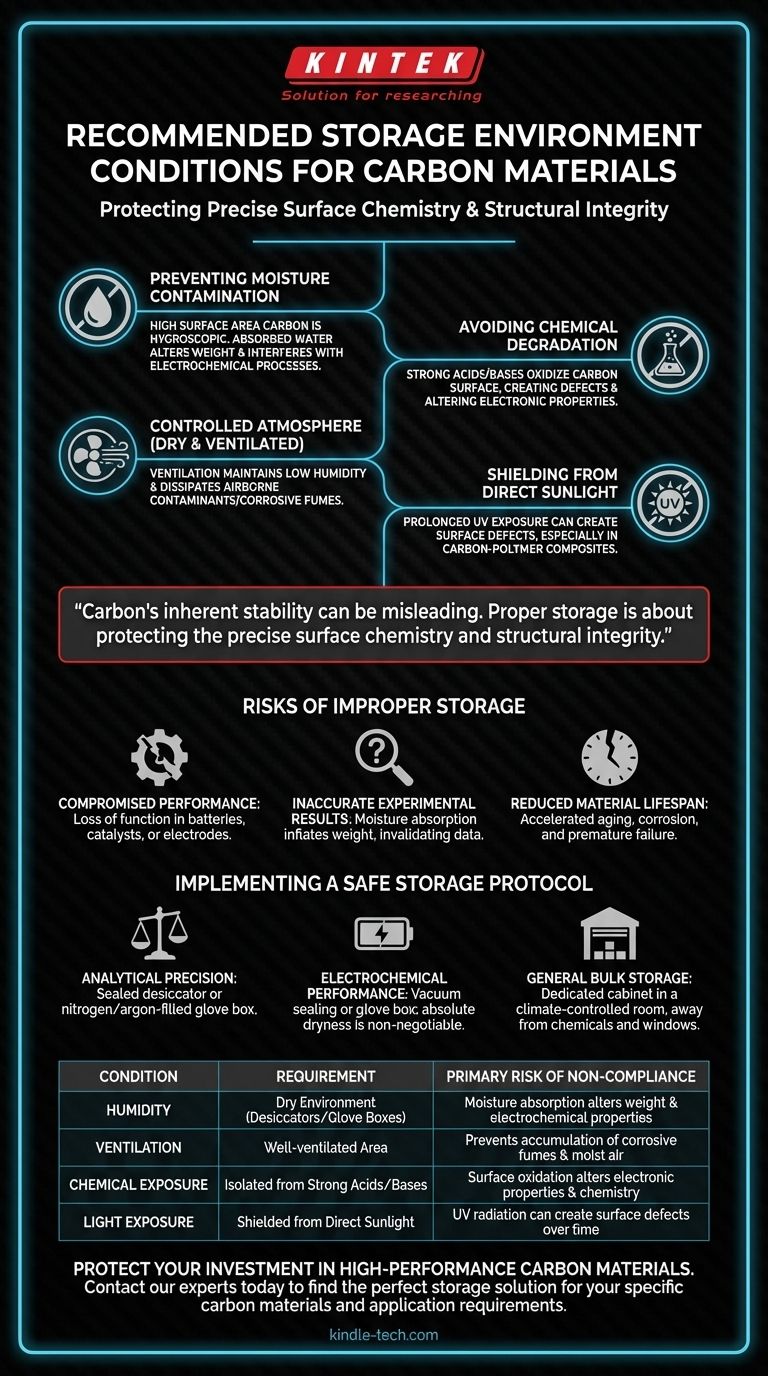
To properly store carbon materials, you must place them in a dry, well-ventilated environment. It is critical to keep them isolated from sources of moisture, corrosive chemicals like strong acids and bases, and direct sunlight to prevent degradation and preserve their essential properties.
Carbon's inherent stability can be misleading. Proper storage is not about preventing the material from disappearing, but about protecting the precise surface chemistry and structural integrity that define its high-performance capabilities.

The Core Principles of Carbon Material Storage
Understanding why these storage conditions are necessary is key to preventing irreversible damage. Carbon materials are often used in applications where even minor surface changes can lead to complete failure.
Preventing Moisture Contamination
Many high-performance carbon materials, such as activated carbon or graphene powders, have a high surface area and can be porous. This makes them naturally hygroscopic, meaning they readily absorb moisture from the air.
Absorbed water can alter the material's weight, leading to inaccurate measurements in research or manufacturing. More critically, it can interfere with electrochemical processes, rendering the material ineffective in applications like batteries, sensors, or supercapacitors.
Avoiding Chemical Degradation
Carbon is generally inert, but it is not immune to attack from aggressive chemicals. Strong acids and bases can react with and oxidize the carbon surface.
This chemical attack doesn't dissolve the material outright but instead creates functional groups or defects on the surface. This alters the material's electronic properties and surface chemistry, which can ruin its performance as a catalyst, electrode, or composite reinforcement.
The Importance of a Controlled Atmosphere
A dry and ventilated environment serves two purposes. Ventilation helps maintain low ambient humidity by preventing stagnant, moist air from settling around the material.
It also ensures that any airborne contaminants or corrosive fumes from other nearby processes are dissipated, rather than accumulating and reacting with the carbon over time.
Shielding from Direct Sunlight
While carbon is highly resistant to heat, prolonged exposure to direct sunlight can be a risk for certain materials. The ultraviolet (UV) radiation in sunlight can, over long periods, provide enough energy to create defects on the surface of some sensitive carbon structures.
This is especially true for carbon-polymer composites, where UV can degrade the polymer matrix, or for highly engineered carbon materials where a perfect structure is essential.
Understanding the Risks of Improper Storage
Failing to follow these guidelines can lead to outcomes that range from inconvenient to catastrophic for your project. The consequences are often subtle and may not be visible to the naked eye.
Compromised Performance
The most common risk is a loss of function. A carbon electrode that has absorbed moisture will perform poorly in a battery. A carbon catalyst with a chemically altered surface will lose its reactivity. These changes are often irreversible.
Inaccurate Experimental Results
If you are using carbon materials for research, moisture absorption will fundamentally compromise your data. Every weight measurement will be inflated by an unknown amount of water, invalidating stoichiometry and concentration calculations.
Reduced Material Lifespan
For components intended for long-term use, improper storage accelerates the material's aging process. Chemical corrosion and UV-induced defects create weak points that can lead to premature mechanical failure or a gradual decline in performance.
Implementing a Safe Storage Protocol
Your storage strategy should match the sensitivity of your application. Use these guidelines to make the right choice.
- If your primary focus is analytical precision: Store materials in a sealed desiccator or a nitrogen/argon-filled glove box to eliminate moisture-related weight errors.
- If your primary focus is electrochemical performance: Absolute dryness is non-negotiable; even trace moisture can impair device function, so vacuum sealing or glove box storage is strongly recommended.
- If your primary focus is general bulk storage: A dedicated cabinet in a climate-controlled room, kept away from chemical storage areas and windows, is sufficient to prevent significant degradation.
By controlling the storage environment, you preserve the inherent value and potential of your carbon materials.
Summary Table:
| Condition | Requirement | Primary Risk of Non-Compliance |
|---|---|---|
| Humidity | Dry environment (use desiccators/glove boxes) | Moisture absorption alters weight & electrochemical properties |
| Ventilation | Well-ventilated area | Prevents accumulation of corrosive fumes and stagnant, moist air |
| Chemical Exposure | Isolated from strong acids/bases | Surface oxidation alters electronic properties and chemistry |
| Light Exposure | Shielded from direct sunlight | UV radiation can create surface defects over time |
Protect your investment in high-performance carbon materials. Proper storage is critical for maintaining the precise surface chemistry and structural integrity required for applications in batteries, catalysts, and composites. KINTEK specializes in providing the reliable lab equipment and storage solutions your laboratory needs to ensure material integrity and experimental accuracy. Contact our experts today to find the perfect storage solution for your specific carbon materials and application requirements.
Visual Guide
Related Products
- Custom PTFE Teflon Parts Manufacturer Corrosion Resistant Cleaning Rack Flower Basket
- Laboratory Vacuum Tilt Rotary Tube Furnace Rotating Tube Furnace
- Vacuum Induction Melting Spinning System Arc Melting Furnace
- Laboratory High Pressure Vacuum Tube Furnace
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
People Also Ask
- What is the procedure for using a PTFE cleaning basket? A 3-Step Guide for Flawless Results
- What are the common specifications and shapes for PTFE cleaning baskets? Maximize Chemical Purity & Process Integrity
- What precautions should be taken regarding the physical handling and loading of a PTFE cleaning basket? Prevent Damage and Ensure Process Integrity
- How should a PTFE cleaning basket be stored when not in use? Maximize Lifespan & Prevent Contamination
- What is the correct way to place items into a PTFE cleaning basket? Master the Art of Perfect, Repeatable Cleaning

















