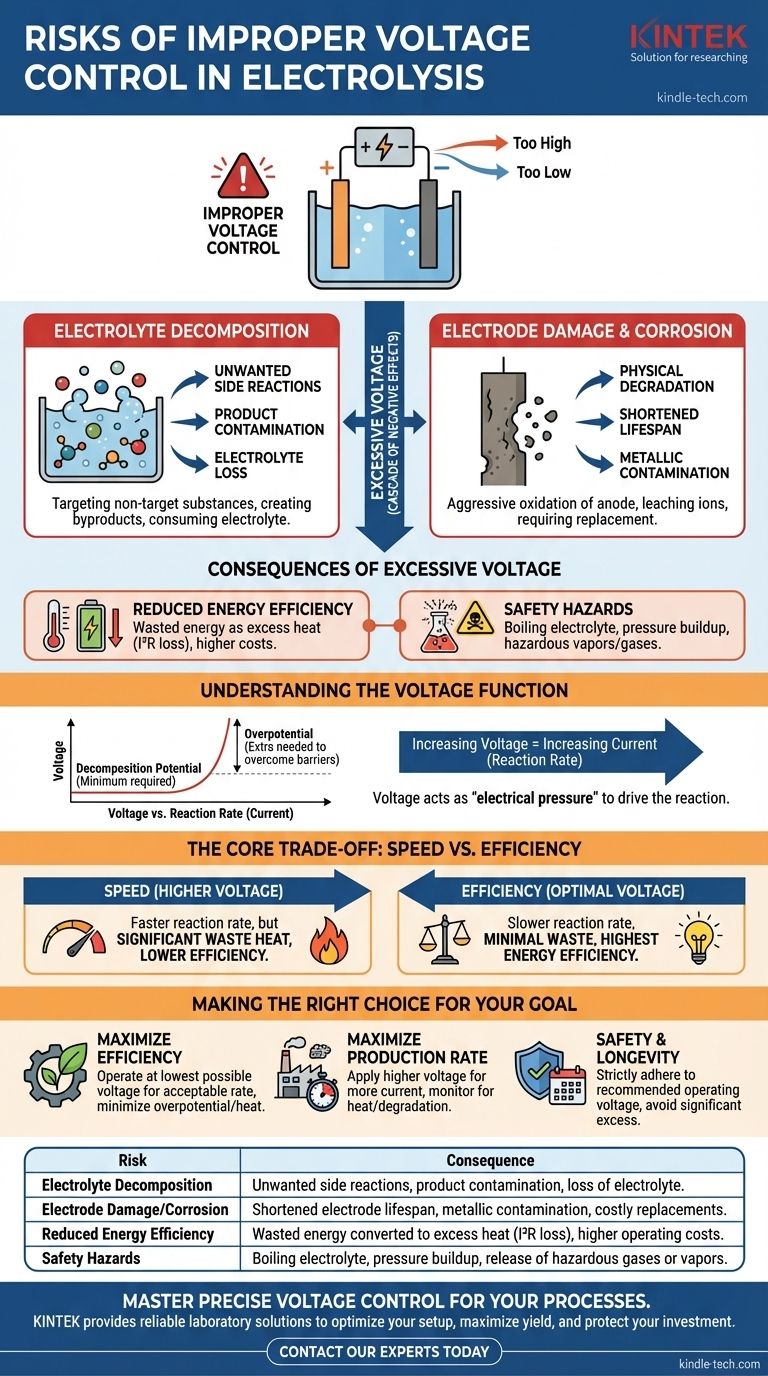
Improper voltage control in an electrolysis cell introduces two primary risks: the decomposition of the electrolyte and physical damage to the electrodes. Applying a voltage that is too high forces unwanted side reactions and can rapidly degrade the cell's components, leading to process inefficiency, contamination, and costly failure.
The central challenge of electrolysis is applying just enough voltage to drive the desired chemical reaction without wasting energy or triggering destructive side reactions. Exceeding this optimal voltage threshold introduces inefficiency, component damage, and potential safety hazards.

The Role of Voltage in Electrolysis
To understand the risks, we must first understand the function of voltage. Voltage acts as the "electrical pressure" that forces a non-spontaneous chemical reaction to occur.
The Decomposition Potential
Every chemical reaction has a minimum voltage required to start it, known as the decomposition potential. Applying a voltage below this threshold will result in no reaction or an infinitesimally slow one.
The Concept of Overpotential
In practice, a voltage slightly higher than the theoretical minimum is needed to overcome kinetic barriers at the electrode surfaces. This additional voltage is called overpotential. The total applied voltage is the sum of the decomposition potential, the overpotential, and any voltage drop due to resistance in the cell (Ohmic loss).
Driving the Reaction Rate
Once the minimum voltage is surpassed, increasing the voltage will typically increase the current. The current is directly proportional to the rate of the reaction—how fast your desired product is being generated. This is why voltage is a primary control lever.
The Primary Risks of Excessive Voltage
Applying a voltage far beyond what is necessary to overcome the decomposition potential and overpotential creates a cascade of negative effects.
Electrolyte Decomposition
Many electrolysis setups use an aqueous electrolyte. If the voltage is too high, you can begin to electrolyze the water itself or other components of the electrolyte solution instead of your target substance. This creates unwanted byproducts, consumes your electrolyte, and contaminates your final product.
Electrode Damage and Corrosion
Excessive voltage can aggressively oxidize the anode (the positive electrode). This corrosion physically degrades the electrode, shortening its lifespan and leaching metallic ions into the solution. This is especially true for less-noble electrode materials.
Reduced Energy Efficiency
The energy efficiency of an electrolysis cell is highest when the applied voltage is close to the minimum required. Any voltage applied beyond this point is largely wasted, converting directly into excess heat. This is known as I²R loss or Ohmic heating.
Safety Hazards
The excess heat generated by high voltage can pose a significant safety risk. It can cause the electrolyte to boil, leading to pressure buildup in a sealed cell or the release of hazardous vapors. Unwanted side reactions can also produce dangerous gases, such as chlorine from a brine solution.
Understanding the Trade-offs
The goal is not simply to minimize voltage but to optimize it for a specific goal, which involves balancing competing factors.
The Problem of Insufficient Voltage
While high voltage is risky, too little voltage is ineffective. Below the decomposition potential, the desired reaction will not proceed. Just above it, the reaction rate (current) may be too slow for any practical application.
Speed vs. Efficiency
This is the core trade-off. Increasing voltage increases the reaction rate (speed). However, as you push the voltage higher, a greater percentage of that energy is lost as waste heat, drastically lowering your energy efficiency. The most efficient operation occurs at the lowest possible voltage that still achieves the desired reaction.
Making the Right Choice for Your Goal
Your optimal voltage strategy depends entirely on what you are trying to achieve with your electrolysis cell.
- If your primary focus is maximizing energy efficiency: Operate at the lowest possible voltage that still provides an acceptable reaction rate, minimizing overpotential and heat loss.
- If your primary focus is maximizing production rate: You will need to apply a higher voltage to drive more current, but you must monitor for signs of inefficiency (excess heat) and electrode degradation.
- If your primary focus is safety and cell longevity: Strictly adhere to the recommended operating voltage for your specific electrode and electrolyte combination, avoiding any significant excess.
Ultimately, precise voltage control is the key to mastering electrolysis, ensuring you achieve your desired outcome safely and efficiently.
Summary Table:
| Risk | Consequence |
|---|---|
| Electrolyte Decomposition | Unwanted side reactions, product contamination, loss of electrolyte. |
| Electrode Damage/Corrosion | Shortened electrode lifespan, metallic contamination, costly replacements. |
| Reduced Energy Efficiency | Wasted energy converted to excess heat (I²R loss), higher operating costs. |
| Safety Hazards | Boiling electrolyte, pressure buildup, release of hazardous gases or vapors. |
Master precise voltage control for your electrolysis processes. KINTEK specializes in lab equipment and consumables, providing reliable solutions for laboratory needs. Our expertise ensures your electrolysis cells operate safely and efficiently, maximizing yield and protecting your investment. Contact our experts today to optimize your setup!
Visual Guide
Related Products
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Optical Water Bath Electrolytic Electrochemical Cell
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
People Also Ask
- What are the typical volumes and aperture configurations for a double-layer water-bath electrolytic cell? Optimize Your Electrochemical Setup
- What is the overall structure of the H-type double-layer optical water bath electrolytic cell? Precision Design for Controlled Experiments
- What are the standard aperture sizes on the lid of the multifunctional electrolytic cell? Key Ports for Your Electrochemical Setup
- What are the standard aperture specifications of the electrolytic cell? Key Sizes for Your Electrochemical Setup
- When is professional repair required for a double-layer water-bath electrolytic cell? Protect Your Lab's Precision and Safety



















