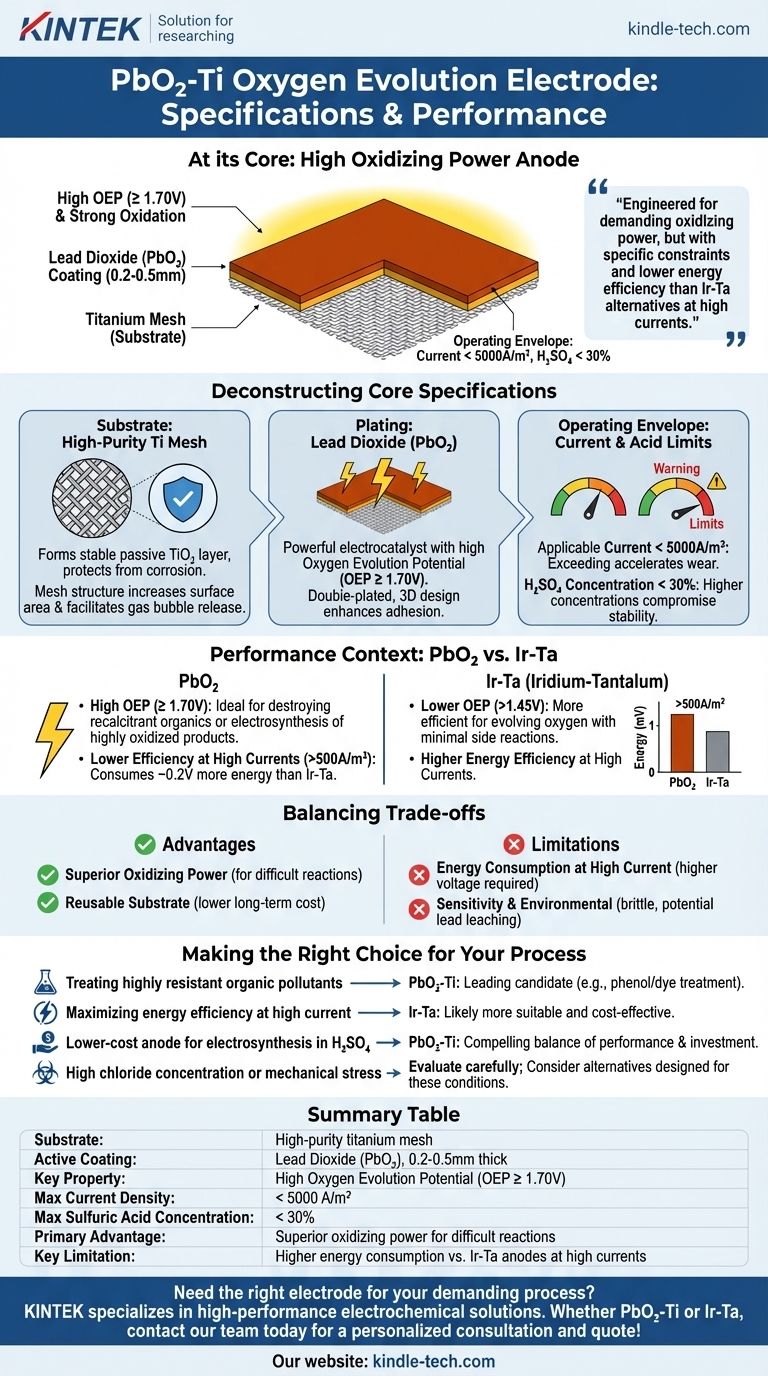
At its core, the Lead Dioxide-Titanium (PbO₂-Ti) Oxygen Evolution Electrode is a specialized anode built on a high-purity titanium mesh substrate. It is coated with a 0.2-0.5mm layer of lead dioxide (PbO₂) and is designed to operate at current densities below 5000A/m² in sulfuric acid concentrations of less than 30%.
This electrode is engineered for applications demanding very high oxidizing power. Its primary advantage is its ability to drive difficult electrochemical reactions, but this comes at the cost of specific operating constraints and lower energy efficiency compared to alternatives like Iridium-Tantalum anodes, especially at high currents.

Deconstructing the Core Specifications
To properly evaluate this electrode, you must understand what each specification means for its performance and durability in a real-world process.
Substrate: High-Purity Titanium Mesh
The foundation of the electrode is a high-purity titanium mesh. Titanium is chosen for its ability to form a stable, non-conductive passive oxide layer (TiO₂), which protects it from corrosion in aggressive electrolytes.
The mesh structure increases the effective surface area, promoting better contact with the electrolyte and facilitating the escape of gas bubbles (like oxygen) from the electrode surface.
Plating: Lead Dioxide (PbO₂)
The active component is the lead dioxide (PbO₂) coating. This is what performs the electrochemical work.
PbO₂ is a powerful electrocatalyst known for its exceptionally high oxygen evolution potential (OEP) of ≥ 1.70V. This high potential is the source of its strong oxidizing capability.
The references note a double-plated, three-dimensional design, which enhances the adhesion of the coating to the titanium substrate, a critical factor for the electrode's service life.
Operating Envelope: Current and Acid Limits
Every electrode has a defined safe operating window. For the PbO₂-Ti anode, these limits are critical.
- Applicable Current (< 5000A/m²): Exceeding this current density can accelerate coating wear and lead to premature failure.
- Sulfuric Acid Concentration (< 30%): This electrode is designed for moderately acidic sulfuric acid environments. Operating in higher concentrations can compromise the stability of both the coating and the substrate.
Performance in Context: PbO₂ vs. Iridium-Tantalum
An electrode's specifications are only meaningful when compared to alternatives. The most common comparison is with Mixed Metal Oxide (MMO) anodes, such as the Iridium-Tantalum-Titanium (Ir-Ta-Ti) electrode.
The Significance of High Oxygen Evolution Potential
The PbO₂-Ti anode's high OEP (≥ 1.70V) makes it highly effective for destroying recalcitrant organic compounds in wastewater or for electrosynthesis of highly oxidized products like persulfates.
In contrast, an Ir-Ta-Ti anode has a lower OEP (>1.45V). It is more efficient for the primary goal of evolving oxygen with minimal side reactions.
A Clear Difference in Energy Efficiency
At low current densities, the energy consumption of a PbO₂-Ti anode is comparable to an Ir-Ta anode.
However, as the current density increases beyond 500A/m², the PbO₂-Ti anode becomes less efficient, consuming about 0.2V more energy than an equivalent Ir-Ta cell. This is a direct consequence of its higher OEP.
Understanding the Trade-offs
Choosing an electrode is an exercise in balancing performance, cost, and operational constraints. The PbO₂-Ti anode presents a distinct set of advantages and limitations.
Advantage: Superior Oxidizing Power
Its primary strength is its ability to facilitate reactions that other anodes cannot. For difficult-to-treat wastewater or specific organic synthesis, this strong oxidizing power is indispensable.
Advantage: Reusable Substrate
Like many high-performance anodes, the titanium substrate is not consumed during operation. Once the PbO₂ coating reaches the end of its life, it can be stripped and the substrate can be recoated and reused, reducing long-term replacement costs.
Limitation: Energy Consumption at High Current
The higher cell voltage required at current densities above 500A/m² translates directly to higher operational energy costs compared to an Ir-Ta anode.
Limitation: Sensitivity and Environmental Factors
Lead dioxide coatings can be more brittle than MMO coatings and may be susceptible to mechanical damage. Furthermore, the potential for lead to leach into the electrolyte if the coating is damaged is a critical environmental consideration that must be managed.
Making the Right Choice for Your Process
Your application's specific requirements will determine if this electrode is the optimal solution.
- If your primary focus is treating highly resistant organic pollutants: The PbO₂-Ti anode's strong oxidizing power makes it a leading candidate for applications like phenol or dye wastewater treatment.
- If your primary focus is maximizing energy efficiency at high current densities: An Iridium-Tantalum (Ir-Ta) anode is likely a more suitable and cost-effective choice in the long run.
- If your process requires a lower-cost anode for electrosynthesis in a sulfuric acid medium: The PbO₂-Ti anode provides a compelling balance of performance and lower initial investment compared to precious metal anodes.
- If your process involves high concentrations of chloride ions or significant mechanical stress: You must carefully evaluate the stability of the PbO₂ coating and consider alternative anode materials specifically designed for those conditions.
Ultimately, selecting the correct anode requires a clear understanding of the electrochemical task, your operating parameters, and the inherent trade-offs of each material.
Summary Table:
| Specification | Details |
|---|---|
| Substrate | High-purity titanium mesh |
| Active Coating | Lead Dioxide (PbO₂), 0.2-0.5mm thick |
| Key Property | High Oxygen Evolution Potential (OEP ≥ 1.70V) |
| Max Current Density | < 5000 A/m² |
| Max Sulfuric Acid Concentration | < 30% |
| Primary Advantage | Superior oxidizing power for difficult reactions |
| Key Limitation | Higher energy consumption vs. Ir-Ta anodes at high currents |
Need the right electrode for your demanding electrochemical process?
KINTEK specializes in high-performance lab equipment and consumables, including advanced electrochemical cells and anodes. Whether you require the superior oxidizing power of a PbO₂-Ti electrode for wastewater treatment or a more energy-efficient Ir-Ta anode for your application, our experts can help you select the optimal solution to maximize your process efficiency and results.
Contact our team today for a personalized consultation and quote!
Visual Guide
Related Products
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Platinum Auxiliary Electrode for Laboratory Use
- Platinum Sheet Electrode for Battery Lab Applications
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- RRDE rotating disk (ring disk) electrode / compatible with PINE, Japanese ALS, Swiss Metrohm glassy carbon platinum
People Also Ask
- What is the difference between RDE and RRDE? Unlock Advanced Electrochemical Reaction Analysis
- What are the performance characteristics of platinum wire/rod electrodes? Unmatched Stability for Your Lab
- What is the common role of a platinum disk electrode? A Guide to Its Primary Use as a Working Electrode
- What is the rotating ring disk electrode method? Unlock Real-Time Reaction Analysis
- What is a common application for the platinum wire/rod electrode? The Essential Guide to Counter Electrodes

















