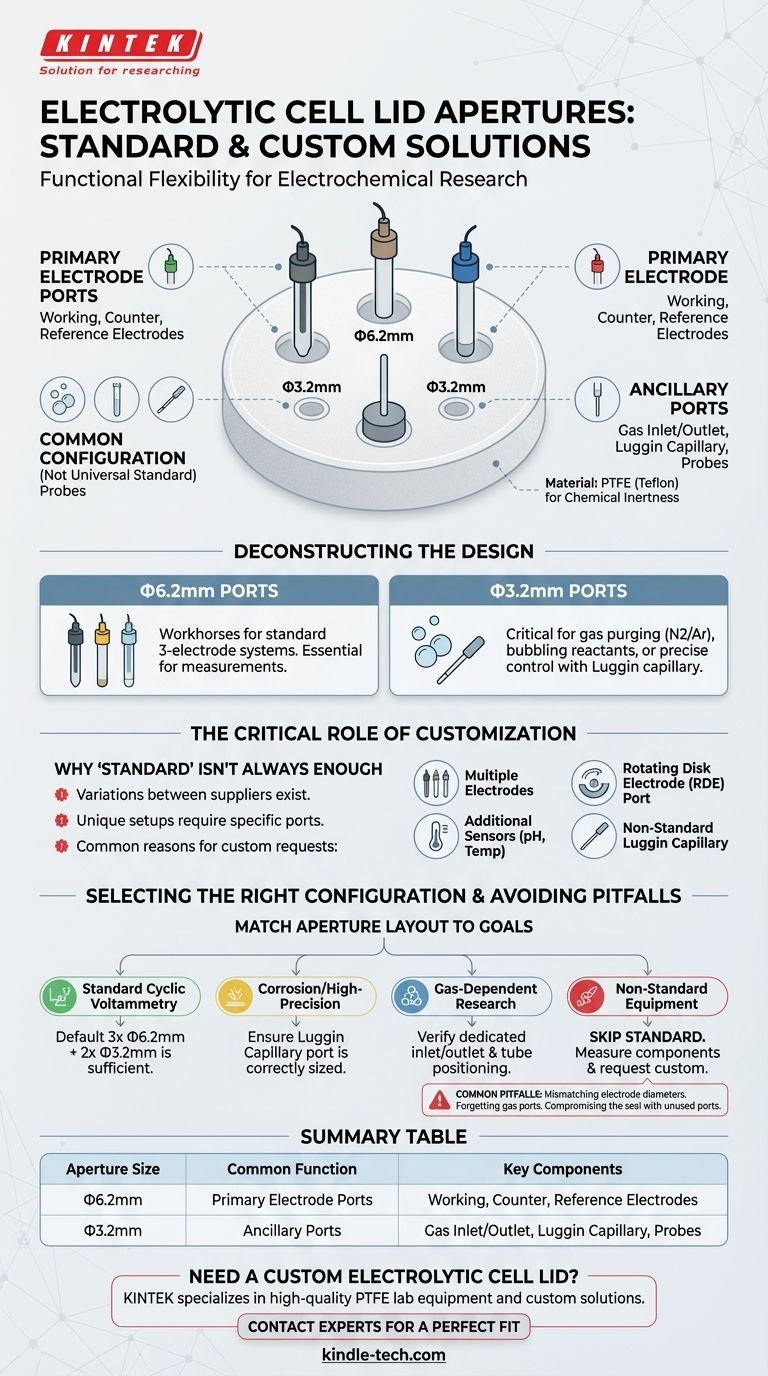
In short, there is no single universal standard, but a common configuration for a multifunctional electrolytic cell lid includes two or three larger apertures of Φ6.2mm and two smaller apertures of Φ3.2mm. These lids, typically made of PTFE, are designed to accommodate the components of a standard three-electrode system, but customization is a frequently used option.
The core principle behind the aperture design is not a rigid standard, but functional flexibility. The configuration is designed to provide the necessary ports for a complete electrochemical experiment while allowing for easy customization to fit specific research needs.

Deconstructing the Standard Lid Configuration
The arrangement of apertures on an electrolytic cell lid is driven by the requirements of the three-electrode system, which is the foundation of most electrochemical measurements.
The Role of the Φ6.2mm Apertures
These larger ports are the primary workhorses of the cell lid. They are sized to fit the bodies of standard working electrodes, counter electrodes, and reference electrodes.
Most experiments require these three core components, which is why you will almost always find at least two or three ports of this dimension.
The Purpose of the Φ3.2mm Apertures
The smaller ports serve ancillary but often critical functions. They are most commonly used for gas inlets and outlets.
This allows an experiment to be purged with an inert gas like nitrogen or argon to remove oxygen, or for reactant gases to be bubbled through the electrolyte. These ports can also be used for smaller probes or a Luggin capillary for precise potential control.
Material and Design
The lid itself is almost universally made from polytetrafluoroethylene (PTFE), also known as Teflon. This material is chosen for its exceptional chemical inertness, ensuring it does not react with the electrolyte or contaminate the experiment.
The Critical Role of Customization
While manufacturers offer "standard" configurations, the reality is that many electrochemical setups have unique requirements. This is why customization is a key feature.
Why 'Standard' Isn't Always Standard
You will notice slight variations in standard offerings between suppliers. One may offer two 6.2mm ports, while another offers three. This reflects different assumptions about the most common experimental setups.
This variability underscores the importance of verifying the configuration before purchase.
When to Request Custom Apertures
Customization becomes necessary when your experiment deviates from a basic setup. Common reasons include:
- Using multiple working or reference electrodes.
- Needing a larger port for a rotating disk electrode (RDE).
- Incorporating additional sensors, like a pH meter or thermocouple.
- Requiring a specifically sized port for a non-standard Luggin capillary.
Common Pitfalls to Avoid
Understanding the configuration is only half the battle. Ensuring compatibility and proper setup is crucial for reliable data.
The Mismatch Problem
The most common mistake is assuming a "standard" cell will fit your specific electrodes. Always measure the diameter of your electrodes and compare them against the cell's specifications before ordering.
Forgetting Ancillary Equipment
Consider everything that needs to go into the cell. If you need to purge with gas, you need at least two small ports for an inlet and outlet. Forgetting a port can halt an experiment before it even begins.
Compromising the Seal
While adding more ports offers flexibility, it can also create more potential leak points. Ensure that any unused ports are properly sealed with stoppers to maintain the integrity of your system, especially when working under an inert atmosphere.
How to Select the Right Configuration
Match the cell's aperture layout directly to your experimental goals.
- If your primary focus is standard cyclic voltammetry: A default configuration with three Φ6.2mm ports (for working, counter, and reference electrodes) and two Φ3.2mm ports (for gas) is typically sufficient.
- If your primary focus is corrosion or high-precision studies: Ensure a port is available and correctly sized for a Luggin capillary to minimize IR drop.
- If your primary focus is gas-dependent research (e.g., O2 reduction): Verify the cell has dedicated gas inlet and outlet ports and that the inlet tube can be positioned correctly.
- If your primary focus is using non-standard equipment: Skip the standard options. Measure all your components and provide a precise list of required aperture diameters to the manufacturer.
Ultimately, viewing the cell lid not as a fixed product but as a customizable platform for your experiment is the key to achieving accurate and repeatable results.
Summary Table:
| Aperture Size | Common Function | Key Components |
|---|---|---|
| Φ6.2mm | Primary Electrode Ports | Working, Counter, and Reference Electrodes |
| Φ3.2mm | Ancillary Ports | Gas Inlet/Outlet, Luggin Capillary, Probes |
Need a Custom Electrolytic Cell Lid?
Ensure your electrochemical experiments run flawlessly with a cell lid configured precisely for your setup. KINTEK specializes in high-quality PTFE lab equipment and consumables, offering custom solutions to fit your unique research requirements—from specific aperture sizes to multi-port configurations.
Contact our experts today to discuss your project and get a perfect fit for your laboratory needs!
Visual Guide
Related Products
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell with Five-Port
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- H Type Electrolytic Cell Triple Electrochemical Cell
People Also Ask
- What is the benefit of using a three-electrode electrochemical cell system for evaluating TiN films? Achieve Precision
- What is the structure of a three-chamber H-type electrolytic cell? Unlock Precision for Complex Electrochemical Reactions
- Why must PEC reactor windows have high mechanical strength? Ensuring Safety and Integrity in Solar Energy Conversion
- What experimental utility does the H-type dual-chamber reactor offer for Algae Fuel Cells? Achieve Precision Research
- How should an all-PTFE electrolytic cell be handled to prevent mechanical damage? Protect Your Investment and Data Integrity
- What are the guidelines for sterilizing the electrolytic cell? Ensure Sterile, Damage-Free Lab Results
- How are the components of a three-electrode electrolytic cell system utilized? Optimize PEC Water Splitting Tests
- What is the purpose of utilizing industrial-grade electrolytic cells and circulation pumps? Expert Scale-Up Guide



















