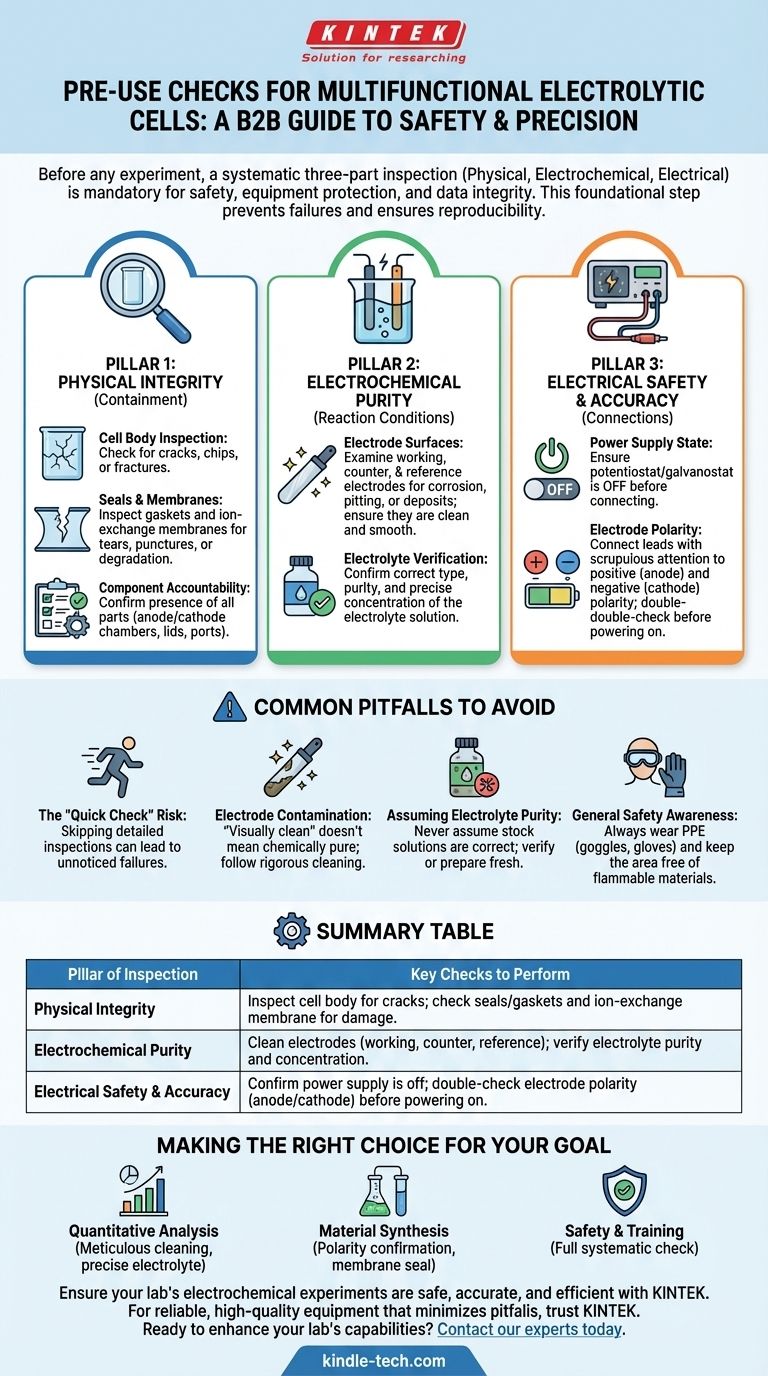
Before initiating any experiment, a systematic inspection of your multifunctional electrolytic cell is mandatory to ensure safety, protect the equipment, and guarantee the integrity of your results. This process involves a three-part verification of the cell's physical integrity, the purity of its electrochemical components, and the correctness of its electrical connections.
The pre-use check is not merely a procedural formality; it is a foundational step in ensuring the safety of the operator and the reproducibility of the scientific data. A failure in any component can invalidate an entire experiment and pose significant safety hazards.

The Three Pillars of Pre-Use Inspection
A thorough pre-use inspection can be broken down into three critical areas. Missing any one of these can compromise your work.
Pillar 1: Verifying Physical Integrity
The physical structure of the cell is your primary containment. Any breach can lead to leaks, loss of material, and potential hazards.
Check the Cell Body The main body, often made of glass or other fragile materials, must be handled with care. Visually inspect it from all angles for any cracks, chips, or fractures. A compromised cell body cannot be used.
Inspect Seals and Membranes Ensure all sealing rings or gaskets are present, pliable, and free from nicks or signs of degradation. If your cell uses an ion-exchange membrane, carefully inspect it for tears, punctures, or discoloration that might indicate aging or damage. A faulty membrane will lead to cross-contamination between chambers.
Confirm All Components are Present Account for every piece of the assembly before you begin. This includes the anode and cathode chambers, lids, ports, and any specialized fittings.
Pillar 2: Ensuring Electrochemical Purity
The accuracy of your experiment depends entirely on the condition of the active surfaces and the solution they are in.
Examine Electrode Surfaces The working, counter, and reference electrodes are the heart of the cell. Their surfaces must be impeccably clean, smooth, and free from any corrosion, pitting, or residual deposits from previous experiments. If necessary, clean or polish them according to established lab procedures for the specific material.
Verify the Electrolyte Select the correct electrolyte for your planned reaction. Critically, you must confirm that its purity and concentration meet the precise requirements of the experiment. Using an incorrect or contaminated electrolyte is a common source of failed or non-reproducible results.
Pillar 3: Confirming Electrical Safety and Accuracy
Incorrect electrical connections are not only dangerous but can also irreversibly damage your electrodes or sample.
Connect the Power Supply Ensure the power supply (potentiostat/galvanostat) is off before making any connections.
Verify Electrode Polarity Connect the leads to the correct electrodes. Pay scrupulous attention to positive (anode) and negative (cathode) polarity. Reversing these connections can cause unintended reactions, ruin your electrodes, and destroy your sample. Double-check every connection before powering on the system.
Common Pitfalls to Avoid
Even experienced professionals can make mistakes. Being aware of common failure points is critical for consistent success.
The Risk of a "Quick Check"
Skipping a detailed inspection of the ion-exchange membrane or seals because they "looked fine last time" is a significant risk. A small, unnoticed tear can completely invalidate hours of work by allowing reactants to mix.
Electrode Contamination: The Silent Killer
A visually clean electrode may still harbor contaminants that can alter reaction kinetics or introduce unwanted side reactions. Always follow a rigorous cleaning protocol between experiments, especially when working with trace-level analysis.
Assuming Electrolyte Purity
Never assume a stock solution is at the correct concentration or free from contaminants. When precision is critical, it is best practice to prepare fresh electrolyte from high-purity reagents or to verify the concentration of an existing stock.
General Safety Awareness
Your personal safety is paramount. Always wear appropriate personal protective equipment (PPE), such as safety goggles and gloves, when handling electrolytes or electrodes. Keep the area around the cell free of flammable materials, as electrolysis can produce flammable gases like hydrogen.
Making the Right Choice for Your Goal
Your specific experimental goal should dictate which checks you emphasize most.
- If your primary focus is quantitative analysis: Meticulous electrode cleaning and precise electrolyte concentration are non-negotiable for accurate data.
- If your primary focus is material synthesis or electrodeposition: Confirming electrode polarity and ensuring the ion-exchange membrane is perfectly sealed is critical to prevent product contamination or failed deposition.
- If your primary focus is safety and training: The full, systematic physical, chemical, and electrical check must be performed without exception to build good lab habits and prevent accidents.
Ultimately, making these pre-use checks an automatic and rigorous habit is the mark of a disciplined and effective researcher.
Summary Table:
| Pillar of Inspection | Key Checks to Perform |
|---|---|
| Physical Integrity | Inspect cell body for cracks; check seals/gaskets and ion-exchange membrane for damage. |
| Electrochemical Purity | Clean electrodes (working, counter, reference); verify electrolyte purity and concentration. |
| Electrical Safety & Accuracy | Confirm power supply is off; double-check electrode polarity (anode/cathode) before powering on. |
Ensure your lab's electrochemical experiments are safe, accurate, and efficient with KINTEK.
A rigorous pre-use check is your first line of defense. For laboratories that demand reliability, KINTEK provides high-quality electrolytic cells, electrodes, and essential consumables designed for precision and durability. Our equipment helps you avoid common pitfalls like electrode contamination and membrane failure, safeguarding your research integrity.
Ready to enhance your lab's capabilities? Contact our experts today to find the perfect electrochemical solution for your specific application—from quantitative analysis to material synthesis.
Visual Guide
Related Products
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- Customizable PEM Electrolysis Cells for Diverse Research Applications
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell Gas Diffusion Liquid Flow Reaction Cell
People Also Ask
- When is professional repair required for a double-layer water-bath electrolytic cell? Protect Your Lab's Precision and Safety
- What are the standard aperture specifications of the electrolytic cell? Key Sizes for Your Electrochemical Setup
- What is the typical experimental system used with a double-layer water-bath electrolytic cell? Achieve Precise Electrochemical Control
- What is the overall structure of the H-type double-layer optical water bath electrolytic cell? Precision Design for Controlled Experiments
- What are the procedures for after using a double-layer water-bath electrolytic cell? Ensure Equipment Longevity and Data Accuracy



















