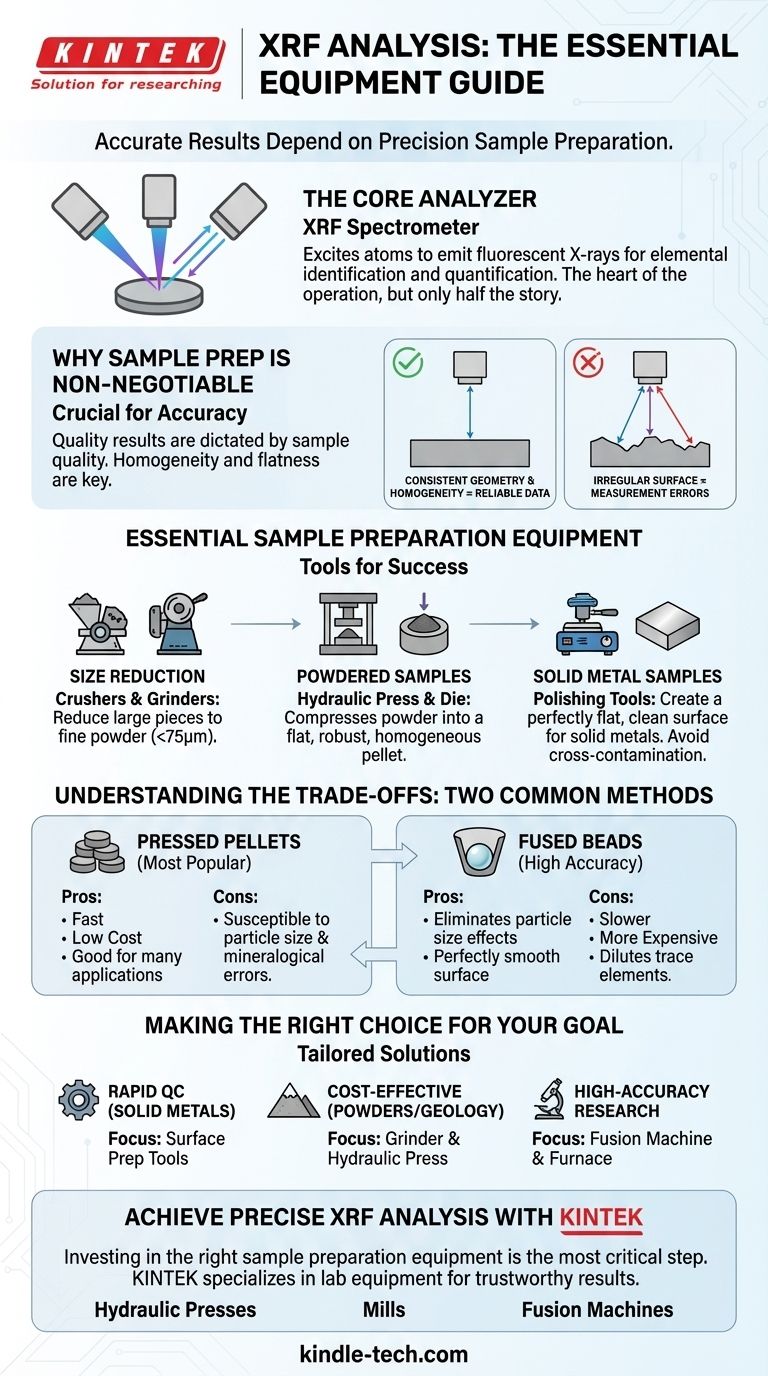
To perform X-ray fluorescence (XRF) analysis, the central piece of equipment is the XRF spectrometer itself. However, to achieve accurate and reliable results, a suite of sample preparation equipment is equally critical, including tools for crushing, grinding, and forming the material into a suitable shape, such as a hydraulic press for making pellets.
The core principle to understand is that the quality of your XRF results is dictated more by the quality of your sample preparation than by the spectrometer alone. The necessary equipment ensures your sample is homogeneous, flat, and truly representative of the material being analyzed.

The Core Analyzer: The XRF Spectrometer
What an XRF Spectrometer Does
The XRF spectrometer is the heart of the operation. It houses an X-ray source (typically an X-ray tube) that excites the atoms within your sample.
When excited, the sample emits secondary, fluorescent X-rays. The spectrometer's detector measures the energy and intensity of these secondary X-rays to identify and quantify the elements present.
Why Sample Preparation is Non-Negotiable
The Principle of Consistent Geometry
XRF systems are calibrated based on a precise, fixed distance between the X-ray source, the sample surface, and the detector.
Any variation in this distance, such as an irregular or non-flat surface, will introduce significant measurement errors because it changes the intensity of the X-rays reaching the detector.
The Need for Homogeneity
The X-ray beam only analyzes a small portion of the sample's surface. For the results to be representative of the entire material, that surface must be perfectly homogeneous.
Without proper preparation, you might be analyzing a single, unrepresentative grain, leading to incorrect conclusions about the bulk material's composition.
Essential Equipment for Sample Preparation
For Size Reduction: Crushers and Grinders
The first step for many materials (like rocks or ceramics) is to reduce them to a fine powder.
Crushers are used for initial size reduction of large pieces. Following this, grinders or mills are used to reduce the material to a consistent, fine powder, typically with a grain size smaller than 75 micrometers.
For Powdered Samples: The Hydraulic Press
Once you have a fine powder, the most common preparation method is creating a pressed pellet.
A hydraulic press and a pellet die set are used to compress the powder under high pressure. This creates a robust, flat, and homogeneous disc that is ideal for analysis. For powders that don't bind well, a wax binder can be mixed in before pressing.
For Solid Metal Samples: Polishing Tools
If you are analyzing a solid piece of metal, you don't need to grind it into a powder.
Instead, you need tools to create a perfectly flat and clean surface. This may include grinding tools, lathes, or polishers. It is also critical to have separate files or cleaning tools for different metal types to prevent cross-contamination.
Understanding the Trade-offs: Two Common Methods
Pressed Pellets
This is the most popular method due to its speed, low cost, and excellent results for many applications.
The primary equipment needs are a grinder and a hydraulic press. However, it can be susceptible to errors from particle size differences and mineralogical effects if not prepared carefully.
Fused Beads
This alternative method involves mixing the sample powder with a flux (like a lithium borate salt) and heating it in a furnace to over 1000°C.
The molten mixture is cast into a glassy disc with a perfectly smooth surface, eliminating particle size effects. While often more accurate, this method is slower, more expensive, and can dilute trace elements, making them harder to detect.
Making the Right Choice for Your Goal
The equipment you need depends entirely on the material you are analyzing and the level of precision you require.
- If your primary focus is rapid quality control on solid metals: Your essential equipment will be surface preparation tools like a lathe or polisher.
- If your primary focus is cost-effective analysis of geological or powdered materials: You will need a grinder to create a fine powder and a hydraulic press to form pellets.
- If your primary focus is high-accuracy compositional analysis for research or certification: You should consider investing in a fusion machine and furnace in addition to grinding equipment.
Ultimately, investing in the right sample preparation equipment is the most critical step toward achieving trustworthy analytical results.
Summary Table:
| Equipment Category | Key Equipment | Primary Function |
|---|---|---|
| Core Analyzer | XRF Spectrometer | Excites the sample and detects fluorescent X-rays to identify elements. |
| Size Reduction | Crushers & Grinders | Reduces sample material to a fine, homogeneous powder. |
| Pellet Formation | Hydraulic Press & Die | Compresses powder into a flat, robust pellet for consistent analysis. |
| Solid Sample Prep | Polishers & Lathes | Creates a flat, clean surface on solid metals for accurate measurement. |
| High-Accuracy Prep | Fusion Furnace | Melts sample with flux to create a homogeneous glass bead, eliminating particle effects. |
Achieve precise and reliable XRF analysis with the right equipment from KINTEK.
Whether your lab focuses on quality control for metals, cost-effective analysis of powders, or high-accuracy research, the quality of your sample preparation is paramount. KINTEK specializes in the lab equipment and consumables you need for trustworthy results, including robust hydraulic presses, mills, and fusion machines.
Let our experts help you build the perfect XRF preparation workflow for your specific materials and accuracy requirements. Contact our team today for a personalized consultation!
Visual Guide
Related Products
- Lab Electrochemical Workstation Potentiostat for Laboratory Use
- Three-dimensional electromagnetic sieving instrument
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Automatic Laboratory Heat Press Machine
- Electron Beam Evaporation Coating Conductive Boron Nitride Crucible BN Crucible
People Also Ask
- What are the limitations of roll bending? Understanding the Trade-offs for Your Metal Forming Project
- Why Use a Laboratory Hydraulic Press for Solid-State Battery Electrolytes? Achieve Maximum Ionic Conductivity
- What is the pressure rating for a hydraulic press? Focus on Tonnage for Maximum Force
- How do laboratory hydraulic presses and molds ensure accuracy in high-strength gypsum concrete testing?
- What are the features of a hydraulic press? Unlock Immense Force with Simple, Reliable Design
- Why is a laboratory hydraulic press required during the preparation of Ti3AlC2 precursor pellets?
- What is the construction of a hydraulic press? The Core Components Explained
- What are three ways to reduce production time in compression molding? Optimize Design, Preheat, and Automate











